Impressive Advances in Treating Chronic Lymphocytic Leukemia

By Kathleen Hoffman, PhD, MSPH
Chronic Lymphocytic Leukemia (CLL) is the most prevalent form of adult leukemia, with about 20,000 new diagnoses per year in the United States alone.1 While it can occur at earlier ages, it is primarily a disease of older adults, with 70 years as the average age at diagnosis. Most of those who are diagnosed have no symptoms.2 CLL is often discovered when patients have a regular CBC test, and the findings indicate that the white blood cell (WBC) count is elevated. Under a microscope, the affected B-cells have a “smudged” appearance.3 The treatment for many patients has been “wait and see,” given that the disease usually progresses slowly over years, even decades.4 Around 30 percent of CLL patients never require treatment.2
When CLL becomes symptomatic including weight loss, extreme fatigue, fever, night sweats, anemia or thrombocytopenia, enlargement of lymph nodes and spleen, and extremely high lymphocyte counts, treatment begins.5 The previous treatment for worsening CLL was chemoimmunotherapy, with a regimen of fludarabine, cyclophosphamide, rituximab (FCR) for younger people and bendamustine, rituximab (BR) for older people or those less tolerant of the side effects of the FCR treatment.6,7 These formerly front-line treatments have been replaced with novel targeted therapies, with impressive results.
In fact, Inspire’s partner, the Leukemia Research Foundation, is having a New and Emerging Treatments Conference next month to discuss these and other recent developments in treating blood cancers. The conference is free and will be virtual. CLL will be covered on October 13th at 7pm (CT).
Identification and mitigation of vulnerable pathways in Lymphocytic B-cell’s have resulted in effective targeted therapies directed at BCR, BCL2, and P13K proteins, as shown below.
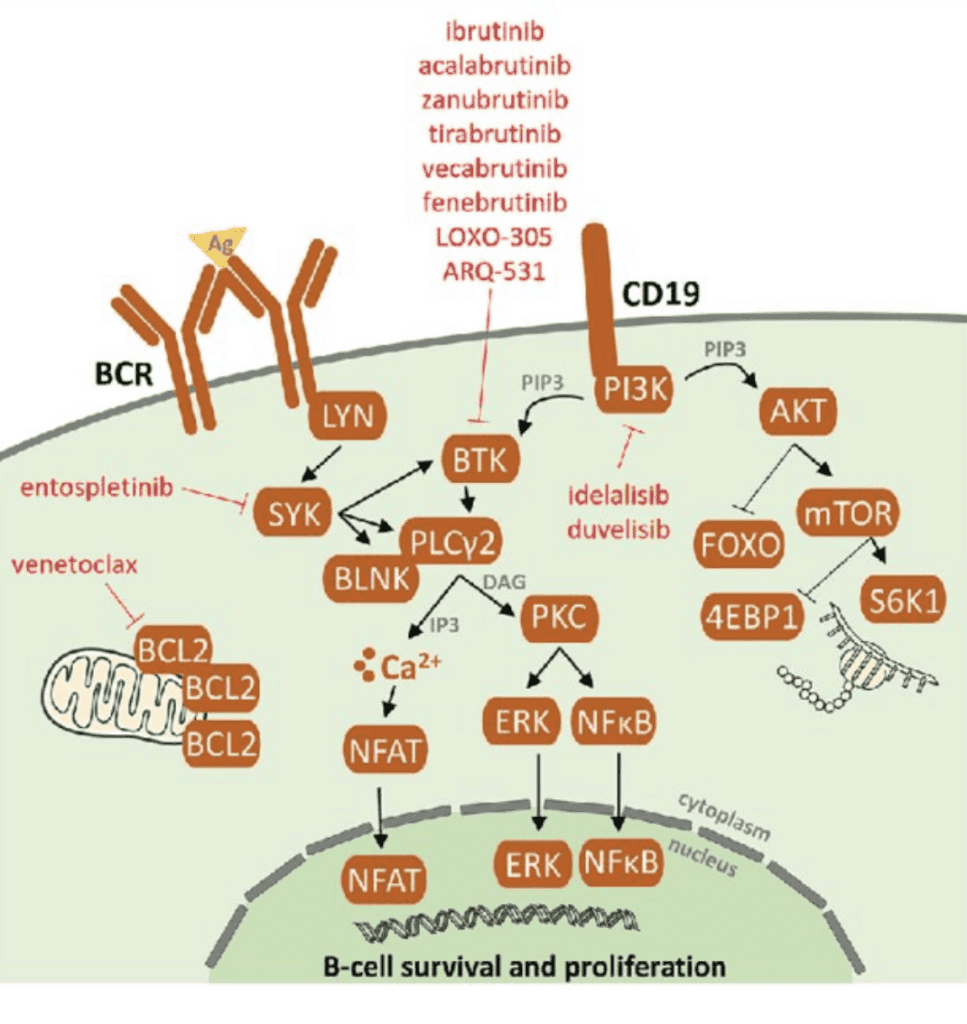
Sedlarikova L.,Petrackova A., Papajik T., et.al.(2020).Resistance-Associated Mutations in Chronic Lymphocytic Leukemia Patients Treated With Novel Agents. Frontiers in Oncology.10. 894. https://www.frontiersin.org/article/10.3389/fonc.2020.00894 DOI:10.3389 8
Because of these advances, a front-line in the armamentarium for CLL, are the B-cell pathway receptor inhibitors and a BCL2 inhibitor8:
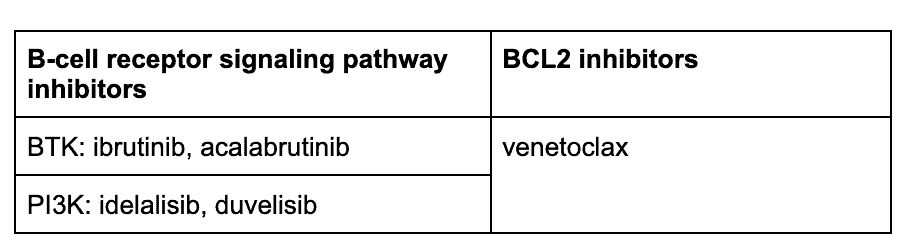
The PI3K inhibitor idelalisib is approved for CLL with demonstrated efficacy and a safety profile, but carries a “black box warning” in its instruction for use because it has been associated with toxicities with unclear causality, such as hepatotoxicity and colitis. Its use requires careful management. Other PI3K inhibitors are being investigated and developed.9,10
BTK inhibitors with or without anti-CD20 monoclonal antibodies
The major players in the BTK inhibitor group are ibrutinib and acalabrutinib, used with or without a CD20 inhibitor such as rituximab or obinutuzumab. One study on ibrutinib with rituximab showed impressive trial results over chemoimmunotherapy: The 3-year progression-free survival was 90.7% vs. 62.5%.11 In fact, ibrutinib works well even in those with a high risk mutations in,11q23 or 17p13, or p53, trisomy 12 and/or un-mutated IGVH genes.
My doctor said that I need to start therapy for CLL. I was diagnosed in [date],, received infusions of [chemoimmunotherapy], and went into remission for 2 years. My CLL began to crawl back, nearing treatment requirements. But… my CLL is in full force and I need immunotherapy… I need to start Ibrutinib/Imbruvica and am looking for personal experiences you all are willing to share. How well have you tolerated it? If it was a problem, did it take months for your body to adjust? Frankly, I would appreciate any experiences you’ve had with it – good and bad.
Ibrutinib can be used continuously as a long-term treatment. A five-year follow up of the RESONATE-2 phase III study comparing ibrutinib with chlorambucil found that patients given ibrutinib had profoundly superior “sustained” progression-free survival (PFS) benefits (70% vs. 12%).12
One characteristic of BTK-inhibitor treatment is that WBC increases dramatically in early treatment, because ibrutinib flushes CLL cells out of the tissues and back into the bloodstream, rapidly shrinking lymph nodes and spleen.13
Bcl-2 Inhibitor: Venetoclax
Venetoclax is a novel treatment. BCL-2 is a mitochondrial protein that inhibits cell death. When venetoclax binds to a lymphoma protein, BCL2, the combination pushes the cancerous cells into cell death (apoptosis).14 A study published in November 2020 characterized venetoclax as providing deep and sustained response in patients with CLL (both new and relapsed cases) and has a well-tolerated safety profile.15 A pooled study of 4 clinical trials concluded that multiple-relapsed patients with CLL showed a response rate of 75% with a complete response rate of 22%. Remarkably, the studies documented clearance of minimal residual disease (MRD) in the peripheral blood in 27% of the patients and in the marrow of 16% of patients. “MRD” is a much higher standard than NED. Participants who had a complete response still experienced progression free survival (PFS) at a 3-year follow up, indicating a deep and durable response.16,17
In contrast with the ibrutinib regimen, where the initial response is a rise in freely circulating WBCs, one of the characteristics of venetoclax treatment is a very rapid drop in the high WBC count. In fact, venetoclax is such an effective antitumor medication that the drug has to be ramped up over five weeks to avoid the adverse effect of tumor lysis syndrome, where the patient is affected by by-products of rapid and widespread cancer cell death.18 Venetoclax can be discontinued after 12 months because of its effectiveness in providing deep remission for the majority of patients.
One Inspire member posted:
Four and a half years in remission via Ibrutinib therapy. CLL is now relapsing. Proposed new treatment regimen is Venetoclax or Venetoclax plus Rituxan. Can anyone share their experience with me?
Better options, better outcomes
The development of the treatment options including the BTK inhibitors and the BCl2 inhibitor, venetoclax, represent major steps forward toward the goal of all patients with CLL: Targeted drugs with long-term, durable results, provided in oral form. The options for CLL patients have broadened, and are becoming more in line with patient QoL desires: Taken at home, and with fewer side effects.
Inspire offers a trusted community to patients and caregivers. Our goal with this blog, this website and our content is to provide the life science industry access to the true, authentic patient voice. In so doing, we support faithful operationalization of patient-centricity. Take a look at our case studies, eBooks and news outlet coverage.
References:
1https://seer.cancer.gov/statfacts/html/clyl.html
2 https://www.ajmc.com/view/cll-overview-diagnosis-prognosis-treatment
3https://pubmed.ncbi.nlm.nih.gov/33527134/
4https://www.nhs.uk/conditions/chronic-lymphocytic-leukaemia/
5https://www.cancer.net/cancer-types/leukemia-chronic-lymphocytic-cll/symptoms-and-signs
6Burger, J.A., O’Brien, S. Evolution of CLL treatment — from chemoimmunotherapy to targeted and individualized therapy. Nat Rev Clin Oncol 15, 510–527 (2018). https://doi.org/10.1038/s41571-018-0037-8
7https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6165790
8Sedlarikova L.,Petrackova A., Papajik T., et.al.(2020).Resistance-Associated Mutations in Chronic Lymphocytic Leukemia Patients Treated With Novel Agents. Frontiers in Oncology.10. 894. https://www.frontiersin.org/article/10.3389/fonc.2020.00894 DOI:10.3389
9https://pubmed.ncbi.nlm.nih.gov/29179250/
10https://doi.org/10.3109/10428194.2015.1022770
11https://www.nejm.org/doi/full/10.1056/NEJMoa1817073
12Burger, J.A., Barr, P.M., Robak, T. et al. Long-term efficacy and safety of first-line ibrutinib treatment for patients with CLL/SLL: 5 years of follow-up from the phase 3 RESONATE-2 study. Leukemia 34, 787–798 (2020). https://doi.org/10.1038/s41375-019-0602-x
13https://ashpublications.org/blood/article/123/12/1772/32694/Lymphocytosis-and-ibrutinib-treatment-of-CLL
14 https://www.hematologyandoncology.net/archives/august-2019/venetoclax-the-first-bcl-2-inhibitor-for-use-in-patients-with-chronic-lymphocytic-leukemia/
15https://ashpublications.org/blood/article/136/Supplement%201/19/473700/Venetoclax-Effectiveness-Safety-and-Treatment
16 https://ashpublications.org/blood/article/134/2/111/260687/Efficacy-of-venetoclax-in-relapsed-chronic
17 https://ashpublications.org/view-large/figure/8446429/blood882555f1.tif
18https://ashpublications.org/hematology/article/2020/1/357/474328/Preventing-and-monitoring-for-tumor-lysis-syndrome
19https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7689538/