Patient and caregiver perceptions of clinical trials: Preliminary findings
By Kathleen Hoffman, PhD, MSPH
Many of you have been in Philadelphia for the “Patients as Partners Conference.” Yesterday, Hannah Eccard, Research manager at Inspire, participated in a panel discussion titled, “Mapping Out the Patient Decision Journey.” The panel discussed the link between understanding patients –their lives, lifestyle, experiences– and trial protocol design.

Recently, Inspire’s research team completed the preliminary analysis of a survey to understand patient and caregiver perceptions and attitudes toward clinical trials. Over 1500 (1644), patient and caregiver members experiencing either ovarian, prostate or colorectal cancer, chronic conditions like psoriasis or arthritis, or rare conditions like scleroderma or sarcoidosis, participated in the study.
There was a high degree of interest in participating in clinical research with 64 percent of patients with these conditions stating that they are interested or very interested in participating in clinical trials.
When asked what words they associated with clinical trials, patients’ lexicon revolved around the promise of clinical trials, with words like hope and cure topping the list. Reflecting that sentiment with actions, almost a third – 31 percent – of all respondents have tried to sign up for a clinical trial. Of this group, half had participated in a clinical trial. This figure is consistent with the results described in Inspire’s Fourth Annual Survey, where around 15% of respondents had participated in a clinical trial.
Support for patients
At the “Patients As Partners” Conference, Hannah’s panel discussion was tasked with several questions. One of these was, “How can pharma help support/provide tools that will help patients while deciding which treatment pathway they choose?”
The preliminary results from our survey suggests that information availability and information complexity are two areas that interfere with patient participation in clinical trials. We found that, of the people who had independently researched clinical trials, 28% had trouble finding information and 21% agreed with the statement “clinical trial information is too confusing.”
Another question the panel grappled with was, “What can sponsors do throughout trial protocols that can help support patients, help alleviate their worries/concerns?”
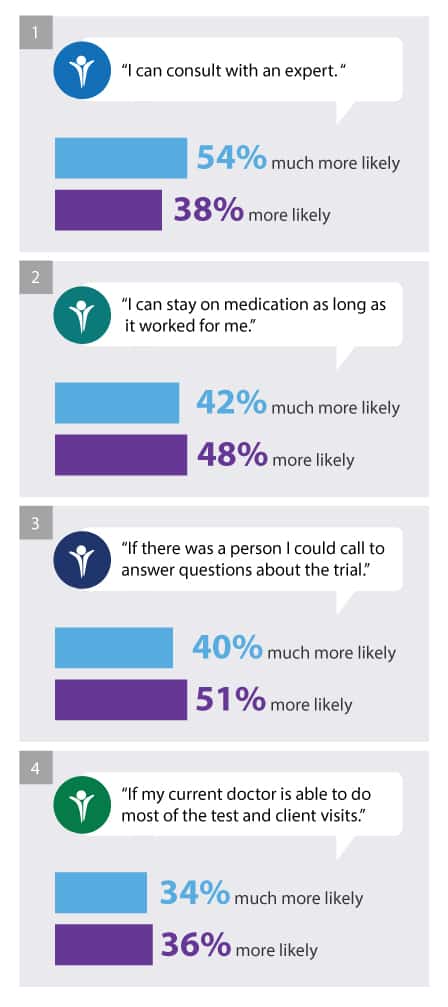
Our survey results contained some insights to answer that question. When we asked patients and caregivers, “How would the following factors influence your likelihood to participate in a clinical trial?” respondents indicated they would be more likely to participate in a clinical trial if
- “I can consult with an expert. “ (54 percent indicated much more likely, 38% indicated more likely,) ;
- “I can stay on medication as long as it worked for me.” (42 percent indicated much more likely; 48 percent indicated more likely)
- “If there was a person I could call to answer questions about the trial.” (40 percent indicated much more likely; 51 percent indicated more likely) ;
- “If my current doctor is able to do most of the test and client visits.” (34 percent indicated much more likely; 36 percent indicated more likely).
Instituting any of these supports could positively influence clinical trial participation and continuation rates.
Patients considerations
Finally, the panel reflected on the question, “What do patients consider when determining to participate/not participate in a clinical trial?”
Although forty-one percent of participants agreed that “Clinical trials feature the newest and best treatments,” our survey found that many respondents only think of clinical trials as an option for “very sick people.” Forty-two percent said that they worry about the safety of the patient in a clinical trial. Twenty-two percent indicated they would only participate in a clinical trial if they had no other options. Eighty-three percent said that, if they were considering a clinical trial, the most important information they would need would be on potential side effects.
These preliminary findings provide some ideas about deterrents and motivators of involvement in clinical trials. What is evident is that to achieve industry goals, industry must focus on the needs of patients. Designing initiatives to address the problems around information complexity, accessibility and safety concerns as well as reassuring patients through contacts and accessible interactions with experts would go a long way toward making patients feel as if they are part of the research, not just “subjects” of the research. This by itself, would be a major step toward improving and increasing interest in clinical trials.
Inspire offers a trusted community to patients and caregivers. Our goal with this blog, this website and our content is to provide the life science industry access to the true, authentic patient voice. In so doing, we support faithful operationalization of patient-centricity. Take a look at our case studies, eBooks and news outlet coverage.