Rethinking patient engagement in clinical trials: Be part of the solution

Seated in the waiting room of a large medical center, I overheard the bright eyed, young man behind the reception desk ask a new patient, “Would you like to learn more about participating in our research?” I smiled and thought, this is part of the solution.
But it is a small part. I’ve been going to the same clinic for five years and today is the first time I’ve heard this question. I wondered if anyone would ask me – a long-term patient – if I would be interested in participating in research.
Participation in clinical research: The statistics
Less than 3% of the population participates in clinical trials, yet when queried, 87% say they are willing to participate if a trial made sense to them.1 A 2018 Landscape Report, “Barriers to Patient Enrollment in Therapeutic Clinical Trials for Cancer” describes the situation this way,
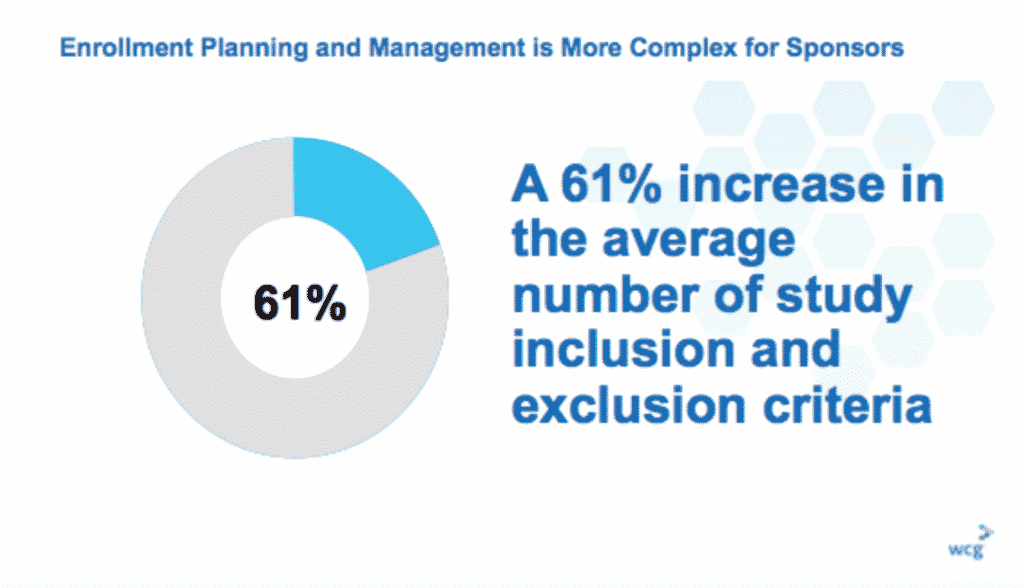
“Enrollment in a cancer clinical trial involves a multi-step process and while participation is typically thought of in terms of a patient decision, it is notable that the patient is not presented with the option until the last step, which is only reached if previous barriers have not been encountered. Analyzing studies across a variety of settings suggests that: • 56% of patients will not have a local trial available for their cancer • 17% will be ineligible for a trial due to exclusion criteria • Many eligible patients will not be asked by their provider to enroll • Only 27% of cancer patients will have the option to enroll in a local clinical trial.” 2
Looking for solutions
In a series of forums, WCG and Inspire are bringing together a variety of viewpoints and perspectives on clinical trials. These breakfast roundtables titled, “Rethinking Clinical Trials: Be Part of the Solution,” are occurring across the country. To increase the patients voice in these discussions, Inspire arranged for patients and patient community representatives to be part of all the forums.
The objectives of the discussions are to determine how to
- “identify and engage high enrolling clinical trial sites”,
- “overcome obstacles in patient enrollment”,
- “tap into online patient communities to open doors to more effective study execution” and
- “partner with patient advocacy groups.” 3
On September 12 and 13, WCG and Inspire held two roundtables, one in San Francisco, the other in San Diego, California. Danya Kaye, Director, R&D and Innovation, represented Inspire at both forums. A slide deck describing the current state of clinical trial enrollment and execution (see below) acted as discussion prompts for the panelists, Kaye said.
Stage 4 Metastatic Colon Cancer, 42 year old mother and registered nurse. $142k insurance premiums?!#IAmAPreexistingCondition pic.twitter.com/zAaedly1jb
— ellen maxon (@amaze_elle) May 5, 2017
San Francisco
For forum contributor, Ellen Maxon, participation in a clinical trial may be the next step in her patient journey. Maxon, an RN at the University of California, San Francisco Medical Center, has stage IV colorectal cancer. Even with the advantage of a medical background, she struggles with the language of clinical trials and the amount of information she must process. According to Kaye, “overwhelming” is the word she used. Indeed, Maxon is not new to the enrollment process; she previously applied for a trial and was determined “ineligible.” Calling attention to the language of clinical trials, she remarked that “the word ‘ineligible’ is offensive and “often makes a patient feel like a failure.”
Along with Maxon, Sharyn Worral, Senior Manager, Education and Research, joined the discussion from Fight Colorectal Cancer, an Inspire partner. (The Fight Colorectal Cancer Support Community on Inspire has over 5000 members.) Her work involves connecting patients to clinical trials and helping them navigate the process.
I lost my mother to Huntington’s disease. @billjohnstonsd is warrior in this fight! We can never give up. Please retweet to raise awareness. #curehd pic.twitter.com/Lgwzvl4BoC — Misty Daniel (@mistyoto) November 24, 2017
San Diego
In San Diego on September 13, Misty Daniel shared her experience as a caregiver. Daniel lost her mother, aunt and brother – over a five year period – to Huntington’s Disease.4 In addition to working with the Huntington’s Disease Society of America at their San Diego branch, she runs clinical trials at Pacific Research Network, a clinical research facility. With intimate knowledge of this disease and experience running a clinical trial, she brought a unique perspective to the roundtable, said Kaye. “Even though you can do everything right from the sponsor’s perspective, it comes down to the human touch at the site level,” Daniel stated. She related the experience of being at a location where a number of competing clinical trials are occurring at the same time. Her takeaway is that, if one sponsor makes it “easier for the staff,” it is more likely staff will funnel patients to that study. In other words, the way the staff are treated at the local level can impact the recruitment numbers.
Future opportunities to attend a roundtable
Two more breakfast roundtables are occurring in the next two weeks. The first is being held in Boston on October 3; the second in Chicago on October 4. Each forum begins at 7:30am with breakfast followed by discussions starting at 8 and ending at 9:30. Attending all of the forums are Jill Johnston, President, Site Support & Management, WCG and Alison Lakin, Associate Vice Chancellor for Regulatory Compliance and Research Integrity Officer, University of Colorado.
Boston
In Boston, Jeff Terkowitz, Vice President of Product at Inspire and Alicia Staley will join Johnston and Lakin. Staley is co-founder of the #BCSM Twitter Chat, a support group started in 2011 for patients and caregivers living with and affected by breast cancer. Meeting on a weekly basis, the #BCSM community has provided an essential online connection for men and women to share the breast cancer patient journey: enduring chemotherapy, celebrating NED and experiencing grief (losing co-founder Jody Schoger along with many others) together.5
Amanda Aikulola, LPN, President and Executive Director of the Dysautonomia Support Network will also be at the panel as an Inspire partner, (the Dysautonomia Support Network on Inspire has almost 1500 members) but also as a patient and health professional. Dysautonomia is the name for a wide range of conditions caused by a malfunction of the autonomic nervous system. Symptoms include fainting, unstable blood pressure, abnormal heart rates, malnutrition, and in severe cases, death. Awareness among the general public is limited; the range of symptoms and the “invisible” nature of the condition can make obtaining a diagnosis a long, difficult process.6
Chicago
In Chicago, Sara Ray, Research Director at Inspire will share the stage with Jonny Imerman, testicular cancer survivor and founder of Imerman Angels. Diagnosed at age 26 in 2001, Imerman had surgery, chemotherapy and a recurrence in the space of a year. During this life-altering experience, Imerman wished he had someone to talk to who was around the same age and had survived the same ordeal. In 2006, he started Imerman’s Angels, a nonprofit that matches “cancer fighters or survivors” with a mentor who has survived the same cancer for one-to-one relationships.7
Joining Ray and Imerman, will be Alexandra Sierra, National Director of Nationwide Research Programs, at the American Lung Association. (In partnership with the Lung Association, Inspire has created several communities including the Lung Association’s Lung Cancer Survivors Support Community on Inspire and the Lung Association’s Living with Pulmonary Fibrosis Support Community on Inspire). In her position she manages the nation’s largest not-for-profit network of clinical research centers dedicated to lung disease research.8
Involving patients in clinical research development
Patients are key to clinical trials and their inclusion in design and development can make an enormous difference in recruitment, enrollment and retention. If unaware of opportunities, distant from trials, barred by time, child caregiving or other “intangibles,” unable to decipher information or overwhelmed by the process, patients won’t be able to participate. If eligibility criteria are too limited, fewer patients can enroll. Accessing the patient voice is crucial to the life science industry to keep clinical research healthy.9
Who, among clinical trial designers, knew that being called “ineligible” would make a patient feel like a failure? Now, because we listened to Ellen Maxon, we know.
Inspire offers a trusted community to patients and caregivers. Our goal with this blog, this website and our content is to provide the life science industry access to the true, authentic patient voice. In so doing, we support faithful operationalization of patient-centricity. Take a look at our case studies, eBooks and news outlet coverage.
Reference:
1 WCG Slide Deck from https://www.eventbrite.com/e/rethinking-clinical-trials-be-part-of-the-solution-san-francisco-tickets-49192352651#
3 https://www.eventbrite.com/e/rethinking-clinical-trials-be-part-of-the-solution-san-diego-tickets-49192845124#
4 https://www.linkedin.com/in/misty-daniel-48800914/
5 https://www.usatoday.com/story/opinion/voices/2016/05/19/voices-powerful-advocate-breast-cancer-survivors-dies/84539844/
6 https://my.clevelandclinic.org/health/articles/6004-dysautonomia
7 https://imermanangels.org/our-founders/
8 https://www.lung.org/about-us/media/media-contacts/alexandra-sierra.html
9 https://www.ncbi.nlm.nih.gov/books/NBK50888/