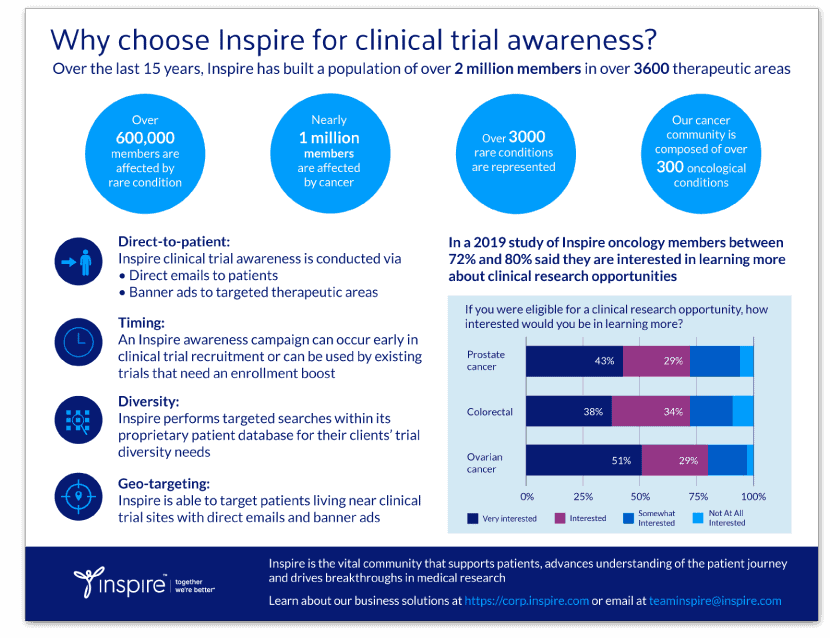
“Still alive and kicking” – The importance of sickle cell disease clinical trial awareness
By Kathleen Hoffman, PhD, MSPH
Sickle cell disease (SCD) is a rare disease: an inherited and incurable blood disorder that causes red blood cells to be misshapen and rigid, affecting their ability to provide oxygen to tissues. Their shape and rigidity cause random blood vessel inflammation and blockages anywhere in the body, with associated organ damage. Six million people suffer from SCD worldwide and many more carry the trait that causes it.1 It prevails in people whose genetics include a connection with sub-Saharan Africa or who come from areas where malaria is, or historically was, endemic.1,2
The primary experience of SCD is profound, life-limiting pain, with the terrible consequence of a shortened life span. In the US, the national average life expectancy is 42-47 years. It is even lower in areas such as sub-Saharan Africa, where 80 percent of the 300,000 babies born annually with SCD live. Childhood mortality in high-burden-SCD countries remains high.3,4
I have sickle cell trait but I feel severe pain all [throughout] my bones all the times. pain is so severe sometimes I will spend months without working.
According to a review of SCD clinical studies by Suarez and others, many SCD clinical trials are terminated because of low enrollment. Pain appeared to be a major negative factor impacting clinical research primarily causing patients to reschedule appointments. This occurred 49 percent of the time in the reviewed studies. Participants reported a pain level greater than zero (0) on at least half of the days during the previous three months.5
Recently approved drugs
Despite having been described over 100 years ago, real progress in treating the disease started with FDA approval in 1998 of hydroxyurea. Since 2017, three more drugs have been approved, adding L-glutamine, crizanlizumab, and voxelotor to the standard of care for SCD. Because of these advances in treatments one Inspire member relayed to others in the community, “Still alive and kicking. To update I am well into my 40s with no signs of slowing down.”
Outside of drugs, hemopoietic stem cell transplant (HST) can even cure the disease, but it is limited by the availability of compatible stem cell donors. There are not enough people of African, Middle Eastern, and South Asian descent in donor registries.6,7
Hope through genomic medicine
Gene editing technologies might be the end-run around the shortage of stem cell donors, as it may enable the perfect donor match: allogenic stem cells from the patient. In this new treatment, the SCD sufferer’s own stem cells are harvested and modified by CRISPR-Cas9 gene editing.8 Chemotherapy is used to eradicate the patient’s own defective hematopoietic stem cells, and the modified stem cells are transplanted back into the patient. These early studies show promise. While studies at one company were halted when two participants developed cancer, the FDA cleared the company to resume trials in June. They agreed with an investigation that showed it was unlikely that the therapy led to the cancer. In fact, one of the two cases turned out to be transfusion-related anemia, and was not the suspected cancer-like bone marrow disease.9
Multiple clinical studies now available due to advancements
A quick search of clinicaltrials.gov reveals that there are 225 studies on sickle cell disease and sickle cell anemia that are in pre-recruitment or recruitment stages. In fact, the NIH is currently recruiting in a clinical trial to study how the PKLR gene affects sickle cell disease.10
Recruiting for SCD trials
In describing the key factors in recruiting and retaining seriously ill African Americans in longitudinal studies, Suarez and others found that the most success in recruiting participants occurred where participants gathered and when they interacted with trusted peers.5 Inspire provides such an environment : a place where patients with SCD and their caregivers come together to share information, and have confidence that they are truly talking with those who understand what it’s really like to live with a disease every day.
Inspire offers a trusted community to patients and caregivers. Our goal with this blog, this website and our content is to provide the life science industry access to the true, authentic patient voice. In so doing, we support faithful operationalization of patient-centricity. Take a look at our case studies, eBooks and news outlet coverage.
References:
1 https://www.nature.com/articles/d41586-021-02255-6
2https://www.cdc.gov/ncbddd/sicklecell/data.html
3 https://www.hematology.org/newsroom/press-releases/2016/rare-patients-with-sickle-cell-disease-live-nearly-twice-as-long-as-average
4 https://bmcmedicine.biomedcentral.com/articles/10.1186/s12916-020-01557-2
5 https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6933076/
6https://www.frontiersin.org/files/Articles/513588/fphys-11-00435-HTML-r1/image_m/fphys-11-00435-g003.jpg
7 https://www.nature.com/articles/d41586-021-02138-w
8 https://www.genengnews.com/insights/viral-or-nonviral-which-is-better-for-crispr-based-therapies/
9 https://www.biopharmadive.com/news/bluebird-resume-gene-therapy-study-fda-hold/601355/
10 https://clinicalstudies.info.nih.gov/ProtocolDetails.aspx?id=2018-H-0146