Continuing Critical Patient-Centric Research Virtually during COVID19
By Richard Tsai
The COVID-19 pandemic is forcing all kinds of businesses and research institutions to find ways of working that provide results similar to what we’re used to gaining from human social interaction. For example, being mindful of the time healthcare professionals need to dedicate to the pandemic has resulted in tremendous delays in HCP-based market research programs. Many clinical trial sites have temporarily suspended work that not only impacts existing enrolled patients but also greatly reduces recruitment. How can we continue critical research programs amidst these challenges? As some companies creatively adapt –administering protocols virtually, sending kits and/ or medical personnel to participants’ homes — patient-focused research, traditionally facilitated by face-to-face in-person meetings, must adapt. When travel is limited and in-person convening cannot occur, what happens to research and how can we continue to engage with patients and caregivers? Are there any insights we can glean from patients to help better prepare business operations and minimize negative impacts for 2020?
Using virtual platforms for research
One unexpected benefit of virtual platforms is that they lower barriers to participation. Recent news articles show that moving meetings and other gatherings to an online setting helps people who couldn’t otherwise participate — belong. In fact, over the last several years we have seen a significant increase in the amount of patient research at Inspire done virtually across many health conditions.
For patient-focused real world real world research, virtual makes sense. According to a publication by the National Academies of Sciences, Engineering, and Medicine,1 factors making a group “hard to study” include “populations that are hard to sample, those whose members are hard to identify, those that are hard to find or contact, those who are hard to persuade to take part, and those willing to take part but hard to interview.” (The same publication notes that, “‘A synonym for ‘hard’ is ‘expensive.”)1 All of these factors and more have always applied to patients with chronic conditions –including people receiving treatments that leave them immunocompromised, such as cancer medications. The CDC notes that these groups are at higher risk of contracting coronavirus.2
How can the move to interacting online help these people? Online, these hard-to-reach groups can participate. They can attend without the hardships created from fatigue, travel expenses, child care expenses, disrupted schedules and the need for help from caregivers. Inspire members, representing a wide variety of conditions, including rare diseases, are widely geographically dispersed, and willing to help each other find ways of getting well.
Over 2 Million Inspire Members
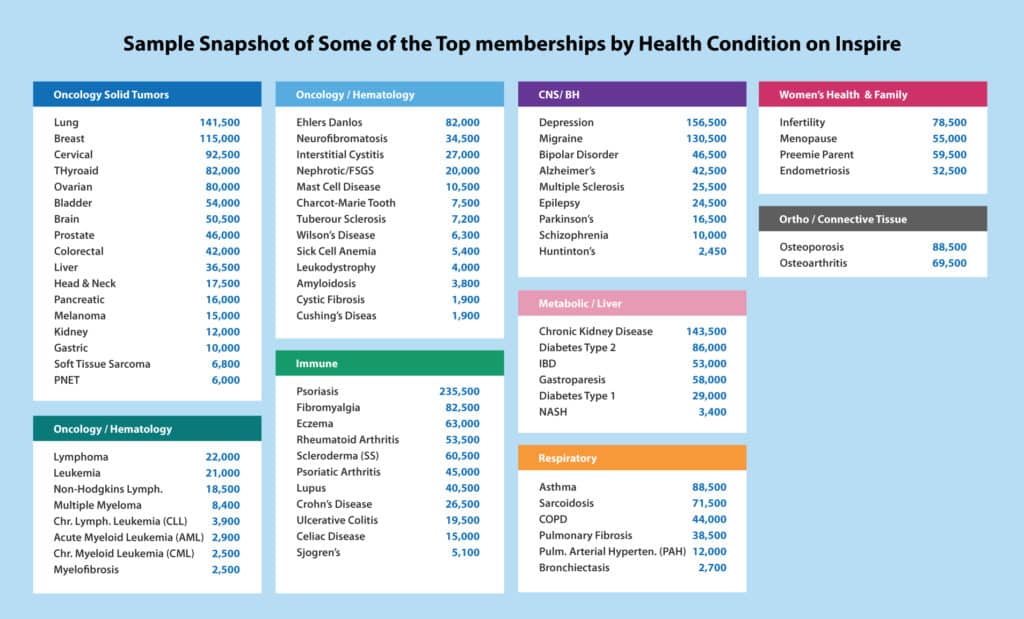
Right now, healthy people are experiencing what life (for years) has been like for some of our Inspire community members — finding that, when getting together in person could be hazardous to your health, getting together online is a welcome resource for both information and human interaction. Researchers gathering online when they can’t attend conferences will find that their “hard-to-study” patient populations have already done so.
Inspire members already know what other groups of people are now trying — sometimes, the very experts you want to learn from are available and sharing information online.
Ascertaining COVID19-related patient insights
Connecting to patients through Inspire can inform the life science industry while they make critical business operating decisions and prioritize commercial marketing activities. Inspire’s online community is discussing COVID19 and our team of researchers are ready and able to conduct sociolinguistic analysis to assess patient language about, and the impact of, COVID19.
Inspire has several other virtual products available to provide patient perspectives when developing patient-engagement related change management plans. One such product is the Virtual Advisory Board (VAB). At a time where patients can’t convene in-person, a VAB is the answer. I invite you to read this week’s case study describing how two pharmaceutical companies used VABs, one for patient-focused R&D, the other for commercial marketing research.
Inspire offers a trusted community to patients and caregivers. Our goal with this blog, this website and our content is to provide the life science industry access to the true, authentic patient voice. In so doing, we support faithful operationalization of patient-centricity. Take a look at our case studies, eBooks and news outlet coverage.
References
1 [unattributed]. “What is a small population?” NASEM. https://sites.nationalacademies.org/cs/groups/dbassesite/documents/webpage/dbasse_183750.pdf. Embedded quote from a study by Tourengeau, R., et. al. (2014). “Hard-to-Survey Populations.” Cambridge University Press
2https://www.cdc.gov/coronavirus/2019-ncov/specific-groups/high-risk-complications.html






