Caregivers: An Untapped Resource for Clinical Research
Caregivers provide substantial support to the health and well-being of patients in the US. One of their contributions involves helping patients search for, and participate in, clinical trials. Caregivers turn to Inspire for help finding and accessing clinical trials.
My father was diagnosed [date] with stage 4 [type] cancer. He has been on numerous rounds of chemotherapy and last scan showed the cancer has progressed…We are now waiting news of any clinical trial he is eligible for…. He is in stable condition now without any issues… We can’t afford to keep waiting until we hear back…. It has been several weeks since his tests were completed and now just waiting. Any and all advice is greatly appreciated…–Inspire member
The process of engaging in clinical trials can be formidable for patients and caregivers. As Renata Louwers, caregiver to her first husband, and now patient and caregiver advocate, wrote:
Patients and caregivers….are utterly overwhelmed when it comes to finding a clinical trial… There are numerous resources to help with the search but simply sorting through the resources can be an enormous task. [They] conduct their own research and do so between chemo appointments, prescription pickups, palliative care pain management consultations, follow up scans, lab appointments, visiting nurse appointments, and blood transfusions. In short, this is a massive workload to place on an already burdened patient and caregiver.1
To learn more about patient and caregiver attitudes toward clinical trials, Inspire’s research team conducted a survey of members in 2019. We shared some of the results in a webinar2 and two blog posts 3,4. Over 1600 members participated, with 109 of those self-identifying as caregivers. These caregivers helped patients with the following conditions: arthritis, colorectal cancer, ovarian cancer, prostate cancer, psoriasis, sarcoidosis and scleroderma.
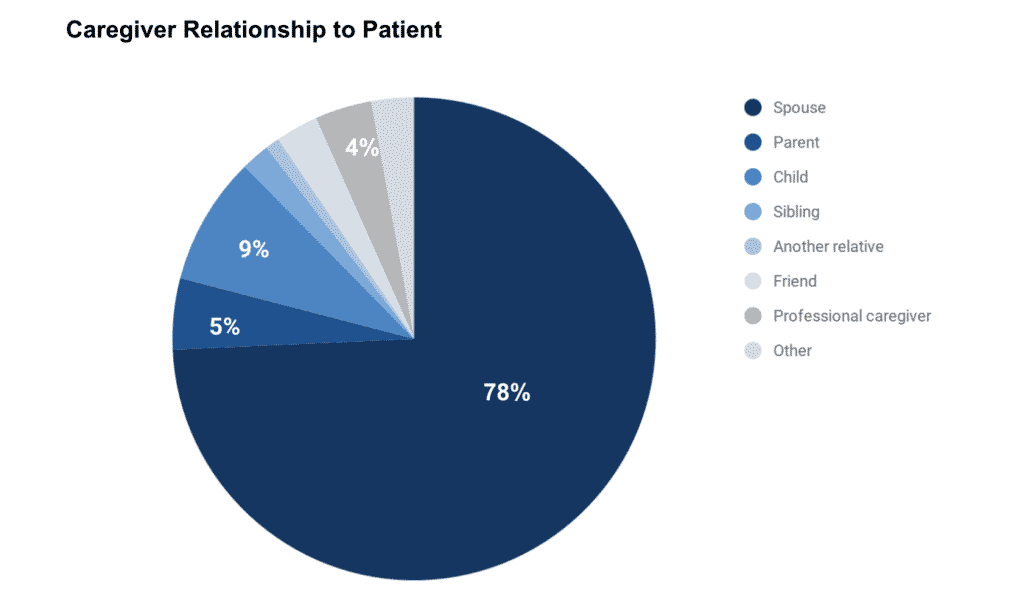
Our survey participant caregivers were predominantly spouses or partners.
These caregivers aid patients in household tasks and give emotional support, but they also perform acute care, monitor medications, and track symptoms.
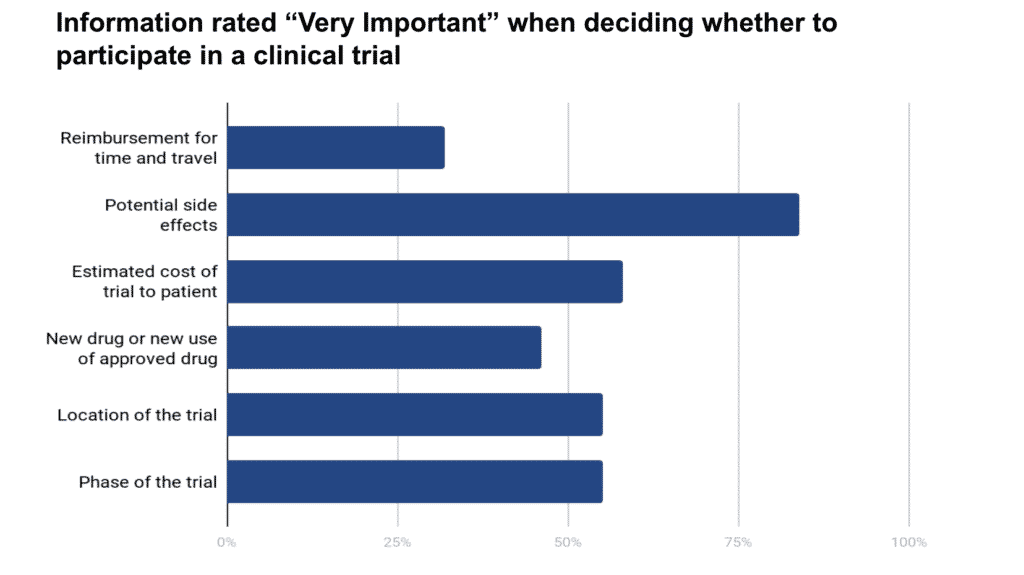
Patients and caregivers often discuss treatment options, including clinical trials, so that both are on the same page when it comes to the patient’s healthcare. When asked, “If your loved one were considering a clinical trial, how important would the following information be when deciding to participate?” potential side effects topped the list, followed by the estimated cost of a trial to the patient.
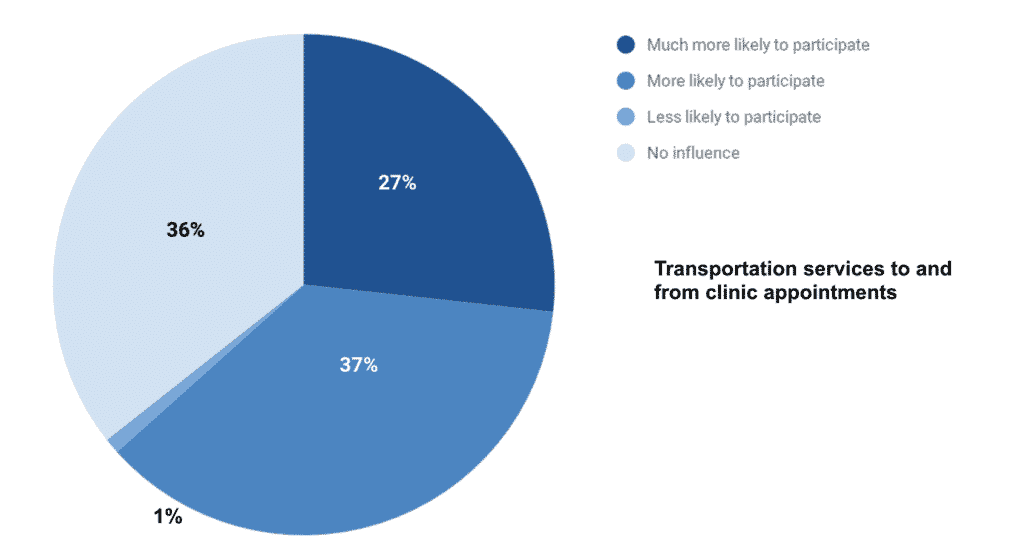
Sixty-four percent of caregivers felt that if the trial had transportation services, they would be much more likely or more likely to participate in a clinical trial.
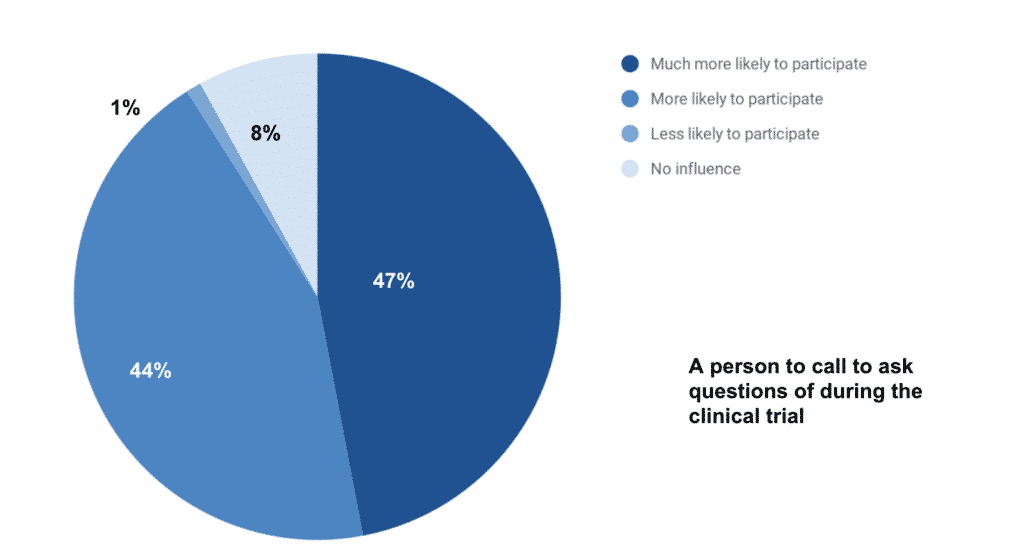
Yet 91 percent (27 percent more) caregivers indicated that they would be more likely to have their loved one participate in a clinical trial if there were a person they could call to ask questions of during the clinical trial.
Caregiver attitudes, understandings, and involvement influence patient participation in clinical trials, but they remain an untapped resource. Caregivers are not only instrumental in clinical trial involvement, they are also another set of eyes observing and participating in the patient journey. They can provide real world information based on their day-to-day experiences, ranging from how medications are actually taken to unmet needs. They observe the care that occurs in the hospitals, with physicians and nurses. They may be instrumental in adherence, know about patients activity levels, cognitive changes, eating, and elimination habits. Caregivers can provide insights into PROs and endpoints.
Bringing the patient voice to clinical trials is a first step to more successful clinical trials. The next step is engaging with caregivers. Explaining clinical trials to caregivers, helping them find clinical trials, and obtaining their insights and experience can help improve clinical trial recruitment and retention and may even support pharmaceutical innovation.
Inspire offers a trusted community to patients and caregivers. Our goal with this blog, this website and our content is to provide the life science industry access to the true, authentic patient voice. In so doing, we support faithful operationalization of patient-centricity. Take a look at our case studies, eBooks and news outlet coverage.
References
1 Louwers, R. (October 26, 2016). Why is it so hard for patients with metastatic cancer to participate in clinical trials? The Philadelphia Inquirer. https://www.inquirer.com/philly/blogs/diagnosis-cancer/Why-is-it-so-hard-for-patients-with-metastatic-cancer-to-participate-in-clinical-trials.html
2Eccard, H. (May 16, 2019). A Hopeful Mindset: Patient Insights on Clinical Trials. [webinar] https://corp.inspire.com/patient-insights-on-clinical-trials
3Hoffman, K. (March 12, 2019 ). Patient and caregiver perceptions of clinical trials: Preliminary findings. Patient Pulse Blog https://corp.inspire.com/blog/patients-caregivers-perceptions
4Hoffman, K. (June 4, 2019 ). Are You Listening to Patients? Patient Preferences and Clinical Trial Design. Patient Pulse Blog https://corp.inspire.com/blog/patient-preferences-and-clinical-trial-design






