[vc_row][vc_column][mpc_textblock content_width=”100″ font_preset=”preset_0″ margin_divider=”true” margin_css=”margin-top:20px;margin-bottom:20px;”]Are You Listening to Patients? Patient Preferences and Clinical Trial Design [/mpc_textblock][vc_row_inner][vc_column_inner][mpc_image image=”11082″ image_size=”large” force_fullwidth=”true” image_opacity=”100″ image_inner_border_gap=”0″ effect=”none” image_hover_opacity=”100″ animation_in_type=”transition.expandIn” animation_in_offset=”100″ animation_in_duration=”1700″ animation_in_delay=”0″][/vc_column_inner][/vc_row_inner][mpc_textblock content_width=”100″ font_preset=”preset_2″ margin_divider=”true” margin_css=”margin-top:20px;margin-bottom:20px;”]By Kathleen Hoffman, PhD, MSPH
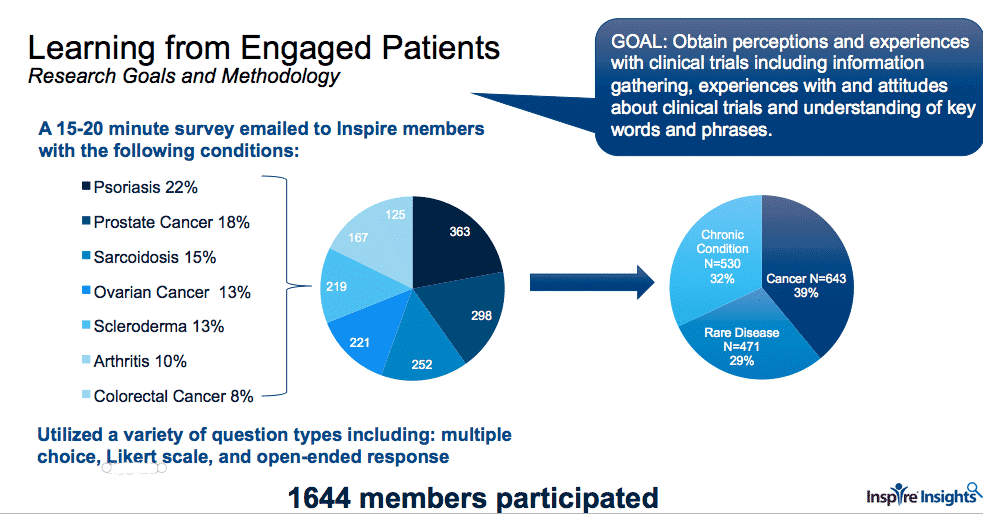
Last fall, over 1500 Inspire members living with sarcoidosis, scleroderma, ovarian cancer, prostate cancer, colorectal cancer, arthritis and psoriasis, completed a survey on exploring patient preferences and insights on clinical trials. Our recent webinar, “A Hopeful Mindset,” described preliminary results. Thirty-nine percent of respondents had cancer, 32% had a chronic condition – arthritis or psoriasis – and 29% had a rare disease.
Over the next few months we will be sharing more details from the data. Last week in “5 Benefits of Patient-Focused Drug Development Sponsors Need to Know,” we provided results across conditions for the question:
“How would the following factors influence your likelihood to participate in a clinical trial? 1)Your current doctor is able to do most of the tests and clinic visits 2) You can consult with an expert in your condition 3)I can stay on the medication as long as it worked for me 4) Transportation services to and from clinic appointment 5) If there was a person I could call to answer questions about the clinical trial” on a 4 point scale from “Much more likely to participate” to “No influence.”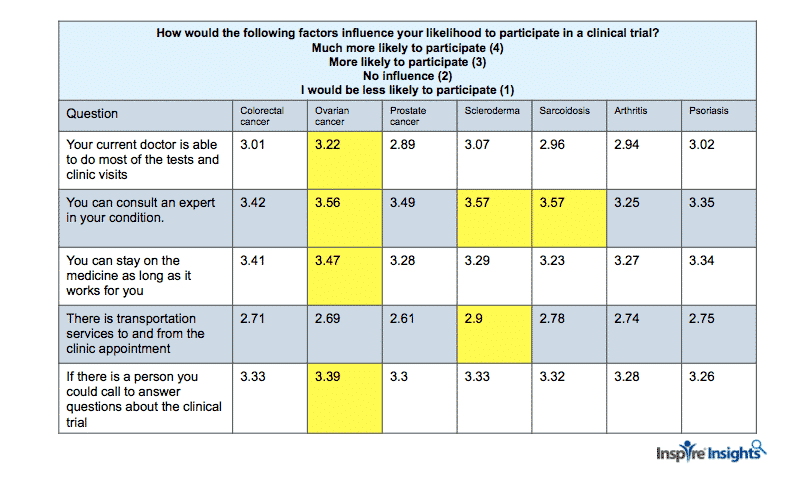
Patient Preferences and Decision-making
Another question elicited the importance of having access to certain types of information during decision-making on participation in a clinical trial. For example, as can be seen in the table below, potential side effects was important information to members across all the conditions, but was most important to members with sarcoidosis. The cost of the trial to the patient was also important, yet it was more important to members dealing with arthritis as their primary condition. Across conditions, those with psoriasis found the location of the trial most important to their decision making.
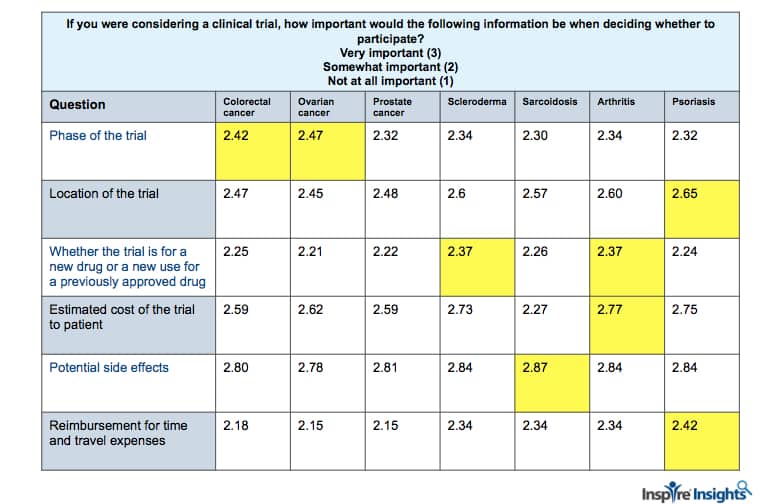
Least important was whether a trial was of a new drug or a new use of a previously approved drug, though across conditions those with scleroderma and arthritis found it more important than other conditions. When considering a clinical trial, the phase of the trial was of greater importance to patients with ovarian cancer and colorectal cancer. Finally, reimbursement for time and travel was most important to the members dealing with psoriasis. (Yellow boxes indicate significance p<=.05.) The results of this question illustrate the importance of learning from patients about their needs and what is important to them before recruiting for clinical trials.
Education and Attitude
Likewise, as we explored demographics, members’ level of education showed significant differences across attitudes to clinical trials.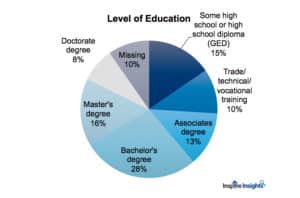
For example, those with some high school or who were high school graduates agreed more strongly with the statement that “The government puts too many restrictions on clinical trials” compared with those who had higher levels of education. People with doctorate level education were less likely to agree with the statement that “If the doctor recommends a trial, it is usually because the treatment is the patient’s best option.”
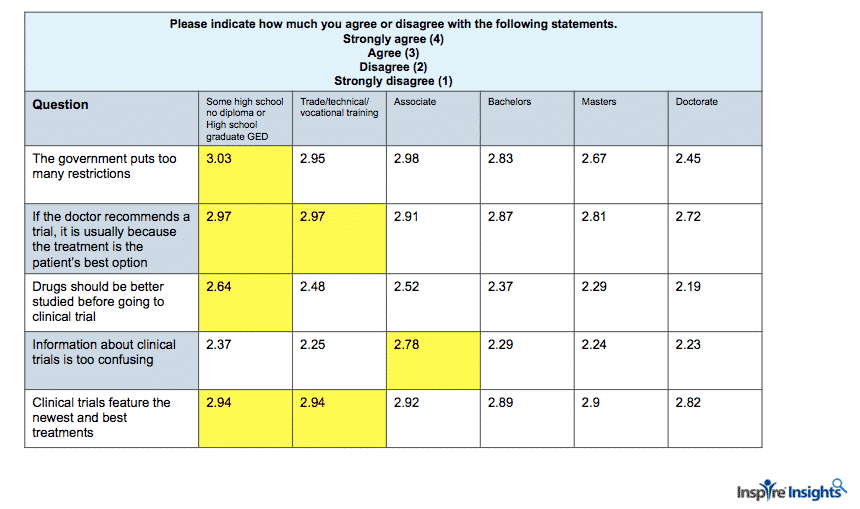
And, the higher the education attained, the less agreement with the statement, “Drugs should be better studied before going to clinical trials.” These differences indicate that certain messaging and appeals may be more effective in recruiting people of different educational backgrounds.
Integrating patient perspectives in clinical trial design and development has become a priority since the 2012 FDASIA reauthorization of the Prescription Drug User Fee Act (PDUFA) and the 21st Century Cures Act. For more on how the patient perspective can improve clinical trial design, please download the case study, What Sponsors Need to Know: Patient Insights on Protocol Design.
[/mpc_textblock][/vc_column][/vc_row][vc_row css=”.vc_custom_1483606399832{padding-top: 20px !important;padding-bottom: 20px !important;}”][vc_column][vc_row_inner css=”.vc_custom_1496685033252{padding-bottom: 40px !important;}”][vc_column_inner width=”2/3″][mpc_textblock content_width=”100″ font_preset=”preset_2″ margin_divider=”true” margin_css=”margin-top:20px;margin-bottom:20px;”]Download the case study, “What Sponsors Need to Know: Patient Insights on Protocol Design.”[/mpc_textblock][mpc_button preset=”preset_1″ block=”true” url=”url:https%3A%2F%2Fcorp.inspire.com%2Fresource%2Fprotocol-design-case-study%2F|title:What%20Sponsors%20Need%20to%20Know%3A%20Patient%20Insights%20on%20Protocol%20Design%20Case%20Study||” font_preset=”preset_1″ font_color=”#ffffff” font_transform=”none” font_align=”center” title=”Download” icon=”fa fa-angle-right” icon_color=”#ffffff” icon_size=”14″ icon_effect=”stay-left” icon_gap=”20″ background_color=”#084a8e” border_css=”border-radius:2px;” padding_divider=”true” padding_css=”padding-top:10px;padding-right:20px;padding-bottom:10px;padding-left:20px;” margin_divider=”true” margin_css=”margin-top:30px;” hover_font_color=”#ffffff” hover_icon_color=”#ffffff” hover_background_color=”#116eba”][/vc_column_inner][vc_column_inner width=”1/3″][mpc_image image=”11051″ image_link=”url:https%3A%2F%2Fcorp.inspire.com%2Fresource%2Fprotocol-design-case-study%2F|title:What%20Sponsors%20Need%20to%20Know%3A%20Patient%20Insights%20on%20Protocol%20Design%20Case%20Study||” image_size=”large” image_opacity=”100″ image_inner_border_gap=”0″ effect=”none” image_hover_opacity=”100″][/vc_column_inner][/vc_row_inner][flipbook-shelf ids=”humanizing_the_brand_experience, insights_from_engaged_patients, expert_by_experience_2016, expert_by_experience_2015, expert_by_experience_2014, case_studies_using_multimodal_research, case_study_prostate_cancer_consumer_support_group_survey, case_study_sleep_disorders_private_research_community”][mpc_callout layout=”style_8″ title_font_preset=”preset_5″ content_width=”100″ content_font_preset=”preset_2″ icon_disable=”true” background_color=”#e8e8e8″ border_css=”border-width:5px;border-color:#d3d3d3;border-style:solid;” padding_css=”padding:15px;” mpc_button__preset=”preset_1″ mpc_button__block=”true” mpc_button__url=”url:%2Fresources|||” mpc_button__font_preset=”preset_1″ mpc_button__font_color=”#ffffff” mpc_button__font_transform=”none” mpc_button__font_align=”center” mpc_button__title=”Learn More” mpc_button__icon=”fa fa-angle-right” mpc_button__icon_color=”#ffffff” mpc_button__icon_size=”14″ mpc_button__icon_effect=”stay-left” mpc_button__icon_gap=”20″ mpc_button__background_color=”#47aff3″ mpc_button__border_css=”border-radius:2px;” mpc_button__padding_divider=”true” mpc_button__padding_css=”padding-top:10px;padding-right:20px;padding-bottom:10px;padding-left:20px;” mpc_button__margin_divider=”true” mpc_button__margin_css=”margin-top:10px;” mpc_button__hover_font_color=”#ffffff” mpc_button__hover_icon_color=”#ffffff” mpc_button__hover_background_color=”#116eba”]Inspire offers a trusted community to patients and caregivers. Our goal with this blog, this website and our content is to provide the life science industry access to the true, authentic patient voice. In so doing, we support faithful operationalization of patient-centricity. Take a look at our case studies, eBooks and news outlet coverage.[/mpc_callout][/vc_column][/vc_row][vc_row][vc_column][mpc_textblock content_width=”100″]
[/mpc_textblock][/vc_column][/vc_row]