Over Two-Thirds of Inspire’s Membership Want Clinical Trial Information
By Richard Tsai
Recently a member of a rare disease community on Inspire asked fellow members,
“What should I know about clinical trials? Are they worth it? Any information would be helpful. Thank you.”
Among the replies they received:
“Unfortunately, we’ve not had many opportunities to participate in [rare disease] clinical trials. In my opinion, if a pharmaceutical company, medical device developer, etc. has gone through the required steps to get to human trials for [rare disease]–I’d absolutely want to help them by participating!”
In another rare disease community on Inspire, a member shared information about a new clinical trial he/she has found.
“There is a new clinical trial of a combination…drug for [rare disease] beginning at [academic medical center] soon. Those who cannot take [medication name]….may consider participating in this trial. I took 6 doses of [the medication] and experienced a [side effect]…I am considering participating in the trial….”
Replies included phrases like, “exciting news!” “please let us know more!” “What’s the name…of the clinical trial so others can look it up?”
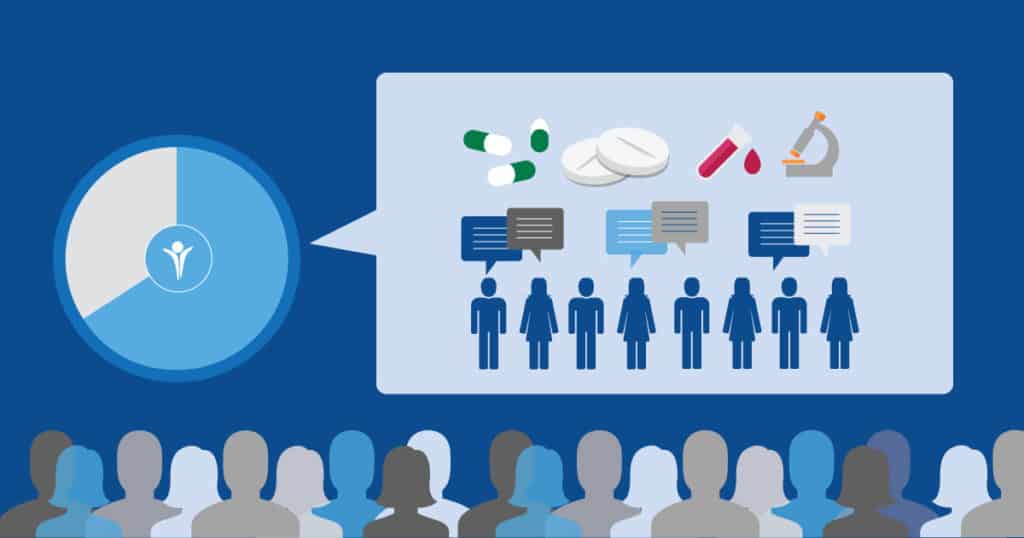
These reactions and this amount of interest are common across Inspire’s communities. Preliminary results from Inspire’s most recent Annual Survey indicate that 69% of our members are interested or very interested in getting information on clinical trials.
Actively searching for clinical trials
Active searches for clinical trials have provided members with cutting-edge, life-saving therapies. For example, in June, we featured Judy Perkins, a member of the Advanced Breast Cancer Support Community on Inspire and the first person to be cured of metastatic breast cancer. Judy’s search for a clinical trial led her to Dr. Steven Rosenberg’s NIH clinical trial program using Tumor Infiltrating Lymphocytes (TILs) for all solid metastatic cancers. Judy continues to share her experience on Inspire and elsewhere, letting other members know about this clinical trial.
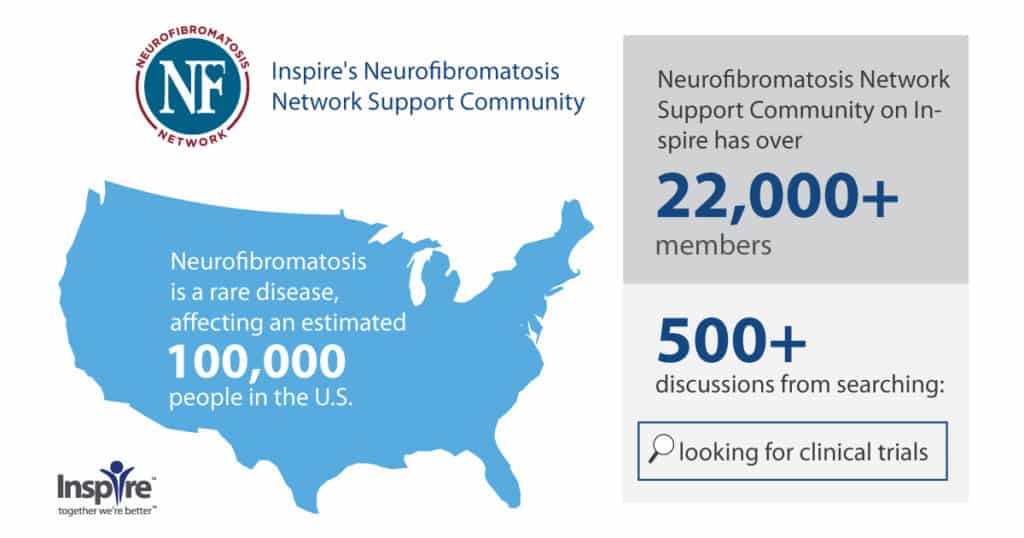
Likewise, motivated for change, research on their condition, and new treatments, Inspire members living with rare conditions seek clinical trial information on Inspire. For instance, the Neurofibromatosis Network Support Community on Inspire, a community representing an estimated 20% of all the people with neurofibromatosis in the US population, had over 500 query matches for “looking for clinical trials.”
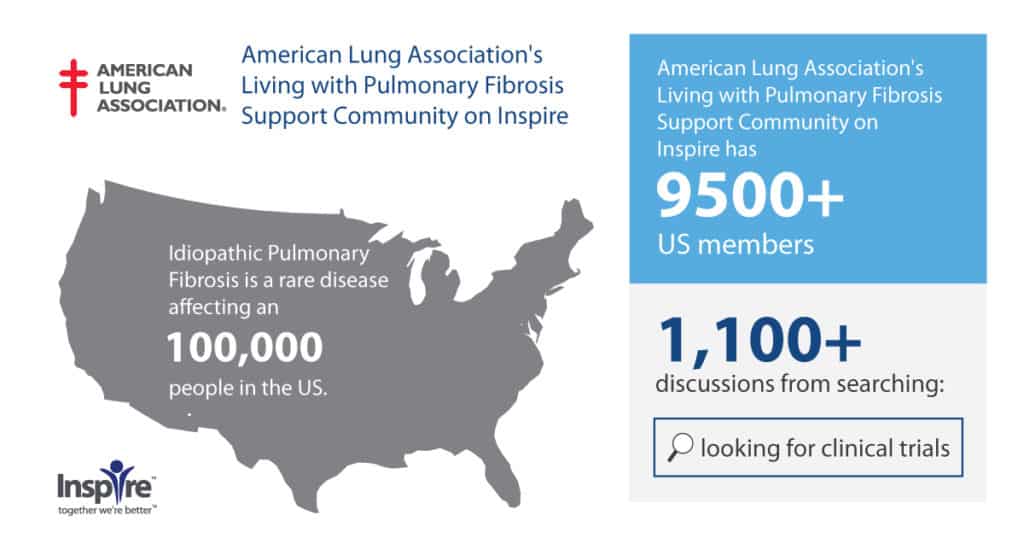
Members living with idiopathic pulmonary fibrosis in The American Lung Association’s Living with Pulmonary Fibrosis Support Community on Inspire has over 1,100 results from “looking for clinical trials.”
Accessing the patient’s perspective
By engaging Inspire’s Research Department, life science corporations can access the insights of patients and caregivers into clinical trial design and development. Government and industry are learning that this crucial step–gathering patient and caregiver unmet needs, discovering barriers to involvement in clinical trials and finding solutions with patients that ultimately increase enrollment and retention–can mean a reduction in costs and an increase in the numbers recruited and retained. The Patient Pulse column, “How R&D Teams Can Utilize Inspire Products for Clinical Trial Optimization” explains some of Inspire’s Research Team’s capabilities and array of tools to provide these insights. See our case studies, “The Virtual Advisory Board Solution“ and “Gaining understanding of the needs of rare cancer patients to enhance clinical trial participation,” to learn more.
New partnership announced
Furthering Inspire’s goals to support patients and advance medical progress, Inspire has partnered with the WIRB-Copernicus Group®’s (WCG™) Clinical Services Division to make clinical research more accessible to our membership. “We are proud to partner with WCG to expand our global network and to deliver even greater value to our existing clients and members,” said Brian Loew, Co-founder and CEO of Inspire in a partnership announcement last week.
Started as the Western Institutional Review Board (WIRB) in 1968, WCG is the first organization dedicated to the ethical review of human research. Its creation preceded by eight years any federal regulation mandating IRB review of human research. Today, WCG provides solutions to improve the quality and efficiency of clinical research. It has relationships with over 2,700 research institutions and almost all of the largest academic medical centers in the US.
In the partnership announcement last week, Donald A. Deieso, PhD, Executive Chairman and CEO, WCG said, “As the focus of clinical research shifts from the treatment of widespread illness to the study of genetic-based disease, patient volunteers are becoming increasingly hard to identify.” To that end, the goal of this partnership is to help Inspire members, especially those with genetically-based and rare conditions, gain greater access to clinical trial opportunities.
On September 12, Inspire will be in San Francisco, CA participating with WCG in a roundtable discussion titled, “Rethinking Clinical Trials: Be Part of the Solution.” Then, on September 13, in San Diego, CA, we will contribute to a second roundtable with WCG: The Future of Clinical Trials: Be Part of the Solution. We welcome all stakeholders involved in clinical trials, from patients to clinical researchers and executives, to register and participate in the forums.
Finally, take a look and download our new case study, “How R&D Can Utilize Inspire Products for Clinical Trial Optimization.” For more information on clinical trial optimization or recruitment, contact us.
Inspire offers a trusted community to patients and caregivers. Our goal with this blog, this website and our content is to provide the life science industry access to the true, authentic patient voice. In so doing, we support faithful operationalization of patient-centricity. Take a look at our case studies, eBooks and news outlet coverage.