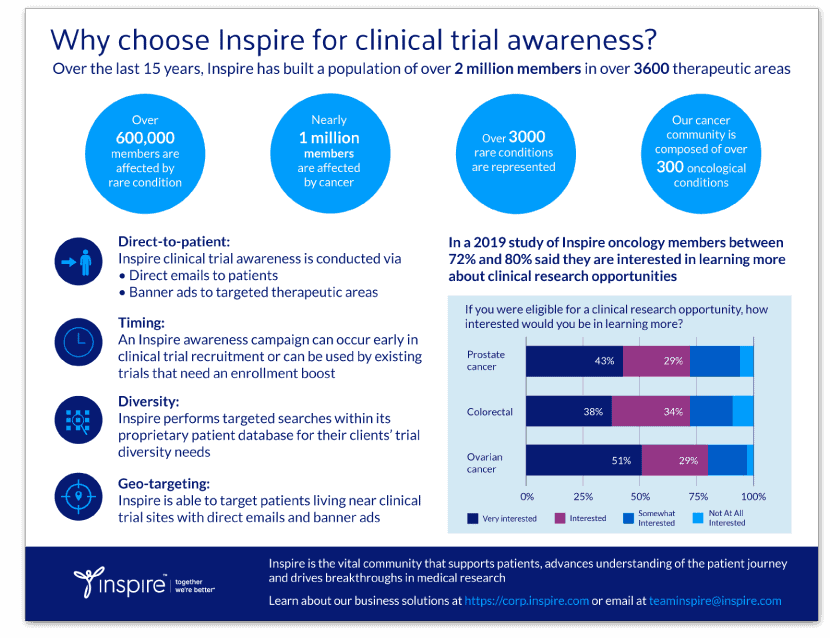
Epilepsy can happen to anyone at any age
By Kathleen Hoffman, PhD, MSPH
Seizures are frightening for those who have them, for their loved ones and for onlookers. Descriptions of epilepsy go back as far as the Sumerians in 2500 BC. Expressing awe and dread, Babylonians, in 1050 BC, called seizures “ṣibtu” translated as “possession” and ṣabātu, “to seize” as in being possessed or seized by the gods.1
Although we still use the term “seizure,” today, these events are now understood. Arising from a disruption in the delicate balance between the internal and external environment of a population of neurons in the brain, seizures are the result of abnormal neuronal firing or excitation. During a seizure, neurons can fire as much as 500 times per second causing involuntary movements and muscle spasms, unusual behaviors and emotions, convulsions, strange sensations, hallucinations, auras and sometimes loss of consciousness.2
The epilepsies are a large group of chronic seizure conditions.
According to the Centers for Disease Control (CDC), around 3.4 million people in the US have epilepsy. The definition of epilepsy has been recently updated to:
1)At least two unprovoked (or reflex) seizures occurring greater than 24 hours apart.
2)One unprovoked (or reflex) seizure and a probability of further seizures similar to the general recurrence risk (at least 60%) after two unprovoked seizures, occurring over the next 10 years.3
As many as 150,000 people receive an epilepsy diagnosis annually.4 Their impact on adults, children and their caregivers should not be underestimated.
I had two or three seizures on [date], including one while driving. I spent two days in the hospital, where an EEG revealed that I have temporal epilepsy. I was put on [medication], which has given me a variety of side effects (will start weaning off [medication] today and begin [new medication]). I had another seizure on Sunday, but luckily was at home and already sitting down when the seizure began. I understand I’m in a period of getting the right medication in the right dosage and that my 6 month no driving period has been extended by my latest seizure….my biggest question concern’s driving. My current job is 180 miles from my permanent home…I have spoken to several medical professionals about staying in my job …and have gotten answers ranging from ‘such a driving schedule is unsafe and unwise ‘to my ‘decision should be made based on my comfort level once the seizures are under control.’ Have any of you had jobs that require long commutes-if so did you stay in your job after your epilepsy diagnosis? Even if I could drive now I’d be terrified to get behind the wheel knowing I could have injured or killed someone when I had the seizures while driving in [date]
My son was first diagnosed with febrile seizures at 15 months old. He had a viral infection with severe fever which lead to a grand mal seizure . The ER doctor sent him home and said it was common in young children to develop febrile seizure when they have high fever. Days following the ER we went to see our pediatrician. And, similarly the doctor said not to worry and is very common in young children to have seizure with fever. Few months later he experienced yet another seizure. To be exact he had multiple seizures in one night which lasted over 30 mins long and the ER diagnosed him with status epilepticus. After that he had multiple seizures that happens every month. We went to see various neurologist. They recommended various testing to conducted , EEG and MRI to his brain. The results for his MRI came back negative, Genetic testing was negative, and EEG showed signs of Complex Partial Seizure with focalized areas of his brain. He is now on medication [medication] was diagnosed with epilepsy…Since he is on [medication] for about 15-18 months now, he has not have a seizure since then. (knock on wood) What should I expect in the future when he is off medication ? The doctor is not 100 % what will happen.
The epilepsies have differing causes but for many (about 50 percent), no cause has been identified.2 Genetics play a role in some of the epilepsies. A study published in October 2021 in the Journal of Neuroscience, describes research into a rare epilepsy caused by mutation in the SCN8A gene. Researchers found that somatostatin interneurons are affected by this mutation. Normally these cells act as brakes to prevent seizures but a genetic change can cause them to actually drive seizures. This discovery provides a cellular target for treatment.3 Disturbances in brain development and wiring, traumatic brain injury from stroke or accident, brain tumors and Alzheimer’s disease are a few of the other causes of epilepsies.4
Epileptic seizures can be classified broadly as either focal – originating in one place in the brain or generalized – originating from both hemispheres of the brain. Medications, devices, diet and surgery have helped between 60 and 70 percent of people with epilepsy. Yet for the 30 to 40 percent who have medication resistant epilepsy, ongoing, uncontrolled seizures can cause brain damage and be life threatening.
Oral anti-seizure medications that are making a difference include cenobamate. Cenobamate addresses two areas in neuron firing, sodium channels and GABA receptors. When compared with placebo, cenobamate reduced seizures by 91 percent.5 Fenfluramine was approved last year for children age 2 and older with Dravet syndrome.6 Approval was extended from focal to general seizures for 2 other medications, lacosamide and perampanel.7,8 Cannabidiol, previously approved for Dravet syndrome, Lennox-Gastaut syndrome, and drug resistant epilepsy, is now approved for seizures occurring in children 1 year and older with tuberous sclerosis.9,10 Brivaracetam has also gotten extended approval for infants aged one month and older for parietal onset seizures.11
Beyond preventive medications there are rescue medicines. The rescue medications midazolam and diazepam, used to shorten seizures, are now available via nasal pump.12, 13
In the last couple of years, deep brain stimulation (DBS) devices have been approved to treat epilepsy. DBS works by sending electrical pulses to the thalamus. In one clinical trial with people who averaged 6 seizures per month, DBS, used over a 7 year period, reduced seizures by 74 percent. Now DBS devices are able to record electrical activity in the brain to help researchers and clinicians learn more about epilepsy.14
Epilepsy can happen to anyone at any age. For the 30 to 40 percent of patients that have resistant epilepsy, new treatments mean hope. On Inspire, members ask each other about treatments and look for answers to bring to their neurologists. Ensuring the best quality of life for all affected, from infants to elders, is the goal. The unpredictability of seizures and possibility of damage to the brain makes the search for treatments of paramount importance.
Inspire offers a trusted community to patients and caregivers. Our goal with this blog, this website and our content is to provide the life science industry access to the true, authentic patient voice. In so doing, we support faithful operationalization of patient-centricity. Take a look at our case studies, eBooks and news outlet coverage.
References:
1Patel, P., & Moshé, S. L. (2020). The evolution of the concepts of seizures and epilepsy: What’s in a name?. Epilepsia open, 5(1), 22–35. https://doi.org/10.1002/epi4.12375
2Office of Communications and Public Liaison National Institute of Neurological Disorders and Stroke, National Institutes of Health. (2018). The epilepsies and seizures: Hope through research: NIH Publication No. 18-NS-156, Bethesda, Maryland
3Journal of Neuroscience 20 September 2021, JN-RM-0718-21; DOI: https://doi.org/10.1523/JNEUROSCI.0718-21.2021
4https://www.mayoclinic.org/diseases-conditions/epilepsy/symptoms-causes/syc-20350093
5Strzelczyk A, Mann C, Willems LM, Rosenow F, Bauer S. Cenobamate for the treatment of focal epilepsies. Expert Opin Pharmacother. 2020 Dec;21(18):2215-2223. doi: 10.1080/14656566.2020.1803830. Epub 2020 Aug 19. PMID: 32812825.
6FDA. (2020). FDA Approves New Therapy for Dravet Syndrome.
https://www.fda.gov/news-events/press-announcements/fda-approves-new-therapy-dravet-syndrome
7Hoffman, M. (2020). Lacosamide Gets FDA Approval for Primary Generalized Tonic-Clonic Seizures https://www.neurologylive.com/view/lacosamide-granted-go-ahead-for-primary-generalized-tonic-clonic-seizures
8https://www.epilepsy.com/medications/perampanel
9Hughes, S. (2020) FDA Approves Cannabidiol for Tuberous Sclerosis Complex
https://www.medscape.com/viewarticle/935067
10Thiele EA, Bebin EM, Bhathal H, Jansen FE, Kotulska K, Lawson JA, O’Callaghan FJ, Wong M, Sahebkar F, Checketts D, Knappertz V; GWPCARE6 Study Group. Add-on Cannabidiol Treatment for Drug-Resistant Seizures in Tuberous Sclerosis Complex: A Placebo-Controlled Randomized Clinical Trial. JAMA Neurol. 2021 Mar 1;78(3):285-292. doi: 10.1001/jamaneurol.2020.4607. PMID: 33346789; PMCID: PMC7754080.
11Antrim, A. (2021). FDA Approves Brivaracetam for Treatment of Partial-Onset Seizures in Pediatric Patients. Pharmacy Times, https://www.pharmacytimes.com/view/fda-approves-brivaracetam-for-treatment-of-partial-onset-seizures-in-pediatric-patients
12https://www.epilepsy.com/article/2019/5/fda-news-nayzilam-midazolam-nasal-spray-approved-seizure-clusters
13https://www.neurelis.com/neurelis-news/neurelis-announces-fda-approval-seizure-rescue-treatment-valtocor-diazepam-nasal-spray-incorporates
14Raven, K. (2021). A New Hope for Patients With Epilepsy. Research & Innovation, Family Health https://www.yalemedicine.org/news/epilepsy-deep-brain-stimulation