COVID-19 vaccine and vulnerable populations: Over 26K participate in ongoing longitudinal study
Crowd-sourcing info for a friend: have any of you who have lupus SLE and/or discoid taken the vaccine? Which one? What were your side effects? Thanks for sharing.
— Brittney Cooper (@ProfessorCrunk) March 9, 2021
COVID-19 vaccines have been released. Are people hesitant about getting vaccinated? How are people responding? How do people with comorbidities, like cancer, psoriasis, asthma or sarcoidosis feel about getting vaccinated? If they have received the vaccines, how have they responded?
It is now possible to find out through Inspire’s COVID HealthJourney dashboard and survey. The dashboard allows people to interactively explore a very large, crowdsourced data set.
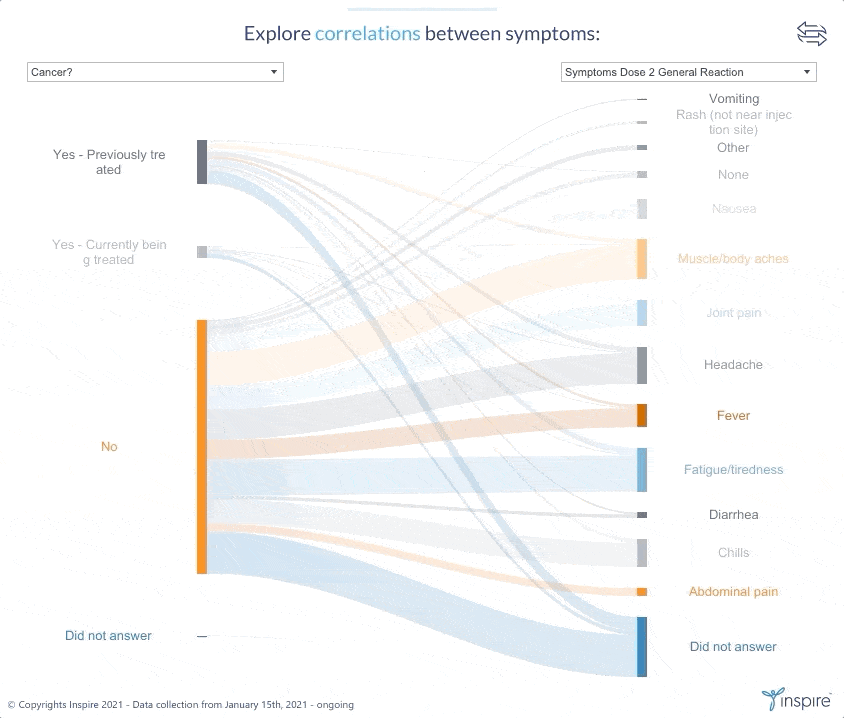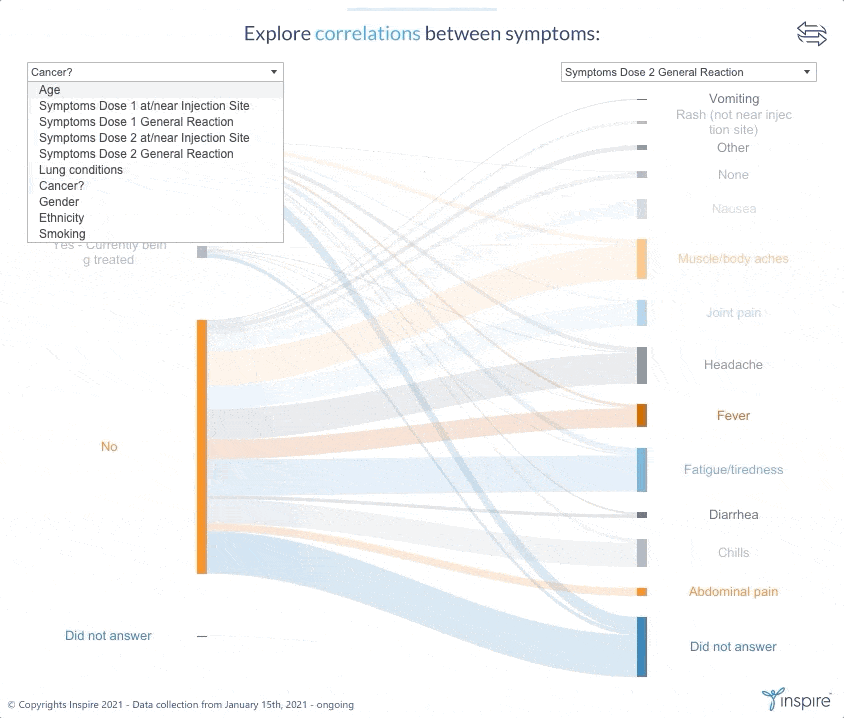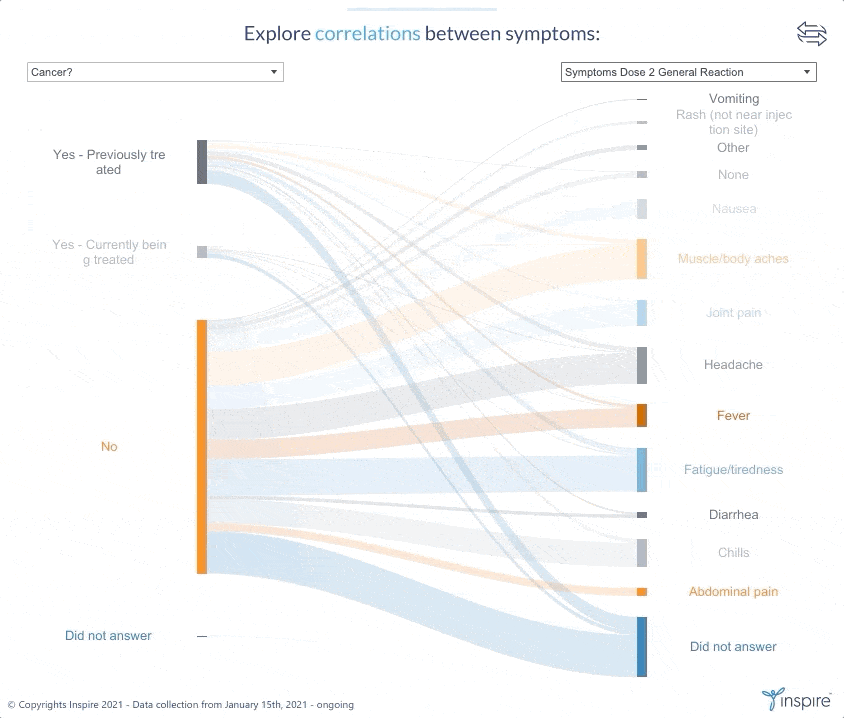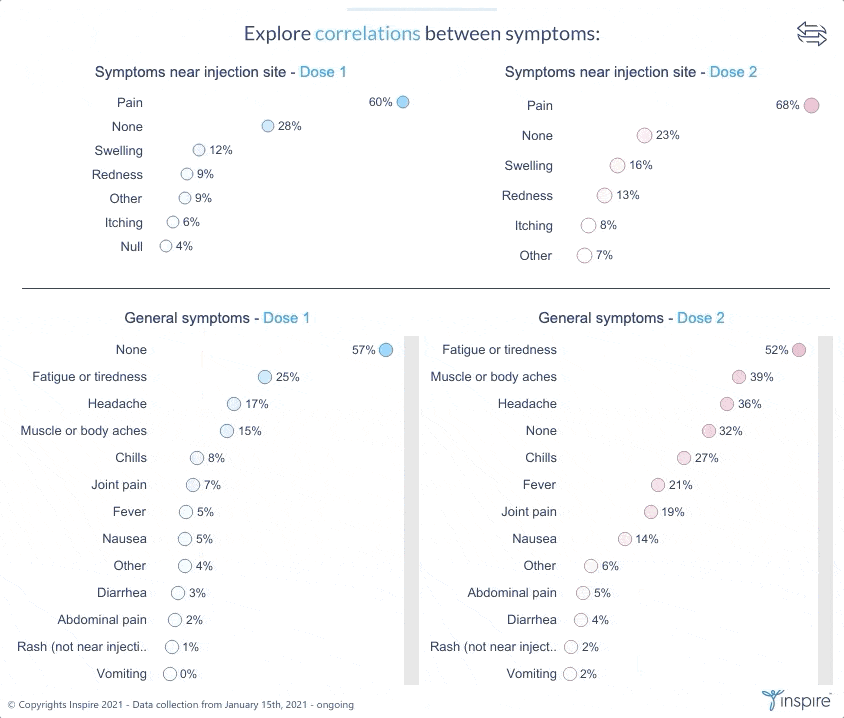
Inspire launched the large-scale survey with members on January 15, 2021 to learn about their experiences and perceptions about COVID-19 and the COVID-19 vaccines. Over 26,000 members have responded to date. Inspire is now sharing its findings (in anonymous form) with members, the public and researchers alike through an interactive Tableau dashboard. The COVID HealthJourney survey is ongoing, and the data will continue to be updated.
The survey addresses three main topics:
- Vaccine willingness, perception and hesitancy
- Vaccine access, experiences, and post-vaccination symptoms
- Long-haul COVID-19: ongoing experiences, symptoms, and recovery
In addition, the survey collects demographics, pre-existing health condition(s), medications, and information on attitudes and behaviors related to COVID-19.
“Until now, there hasn’t been a great deal of information available about how COVID-19 vaccines interact with underlying health conditions,” says Brian Loew, CEO and founder of Inspire. “Inspire is an ideal space to explore this by ‘crowdsourcing’ — learning from one another.”
Inspire’s COVID HealthJourney interactive dashboard provides visual tools for users to explore the data with customized search criteria. Any researcher or consumer will be able to explore vaccine experiences and perceptions filtered by medical condition, age, gender, location, and vaccine type, among others. For example, users will be able to explore the differences between side effects from the Moderna and Pfizer vaccines.
Vaccine willingness, perception and hesitancy
Of the tens of thousands of responses, the majority of respondents (82%) said they intend to get or have already gotten the vaccine, as reflected by this member’s thoughts:
I believe in vaccines. I believe that science and the research that has been put in to save lives. I’m sitting here thanking god right now, because I received my first part of my vaccine in December. A family member has covid now and I’m negative. I believe it saved my life, as I have medical problems that could’ve increased the severity of COVID.
Only 18% of respondents with chronic diseases showed any hesitancy in getting the vaccine. Some worried that the vaccine was too new (50% of those who were hesitant) or were concerned that politics had played a role in its development (39%).
In early February around 10% of the participants were actively trying to get the vaccine but had been unable to obtain it. One person wrote that they had had “difficulty getting a vaccine, which is frustrating…healthcare is not available except for those with internet access.” But others are hopeful,
When the good news came that a vaccine was found it seemed to lift my spirits greatly. I am hopeful for the future now. We are living through history, and will tell future generations……’Yes, I was there when it happened and this is my story.’
Vaccine experiences and symptoms
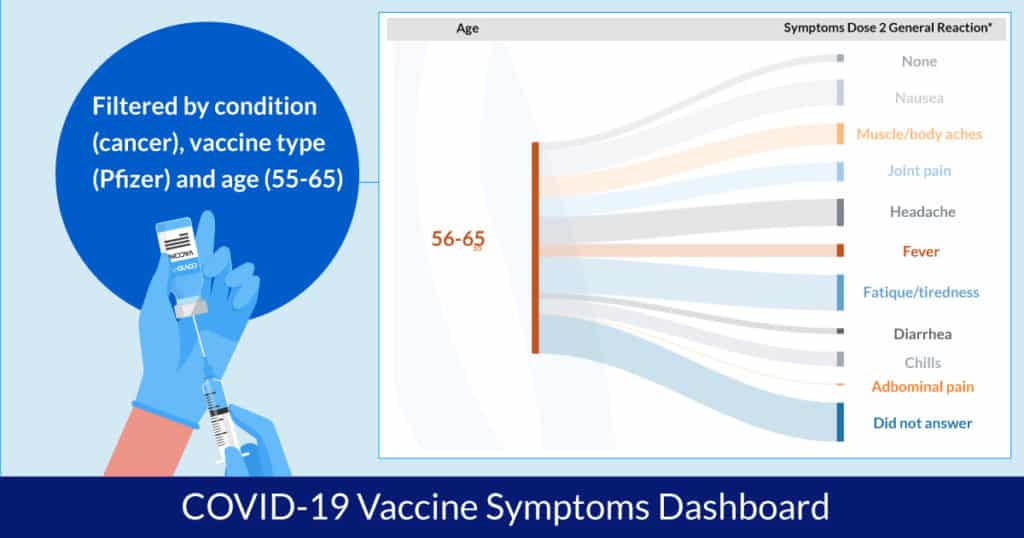
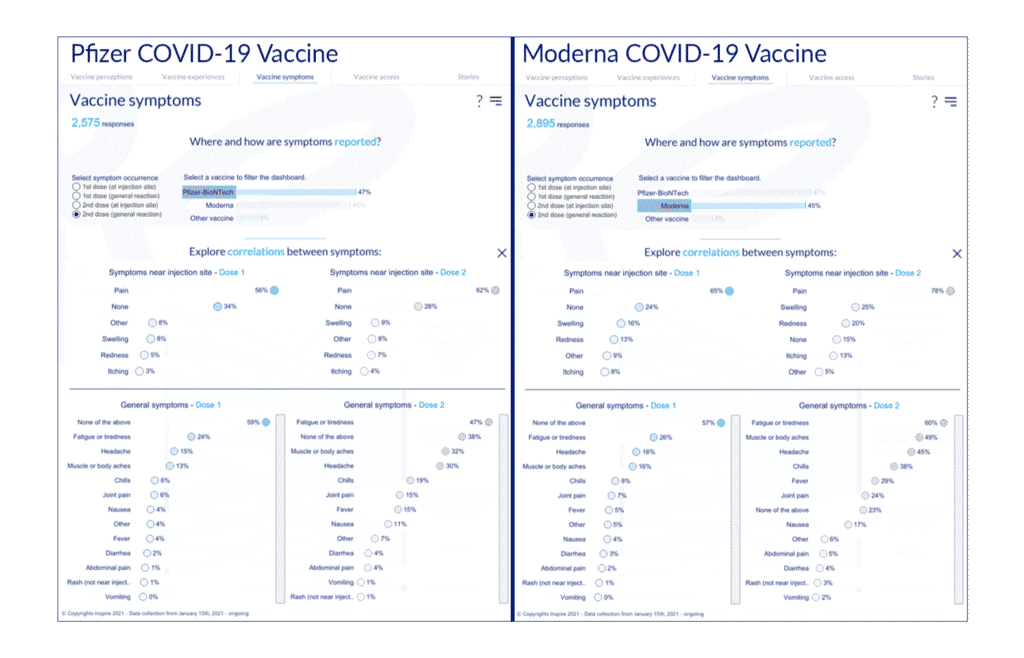
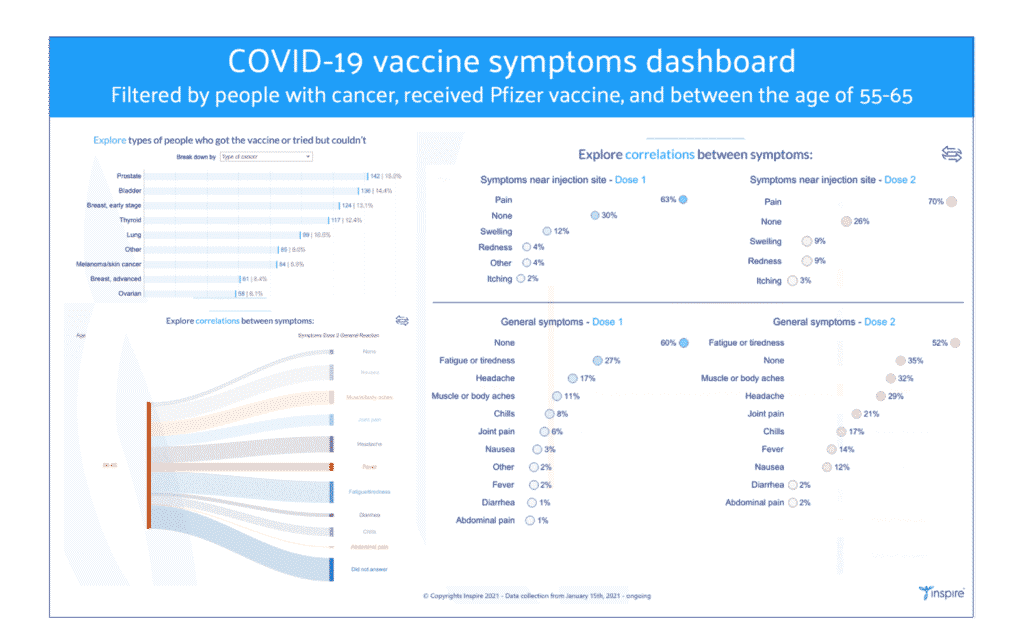
There are millions of permutations that can be created through this dashboard, depending on your question. Here is a step-by-step description. Suppose you would like to learn the responses of those who have or have had cancer, have received the COVID-19 vaccine manufactured by Pfizer/BioNTech and are age 55-65.
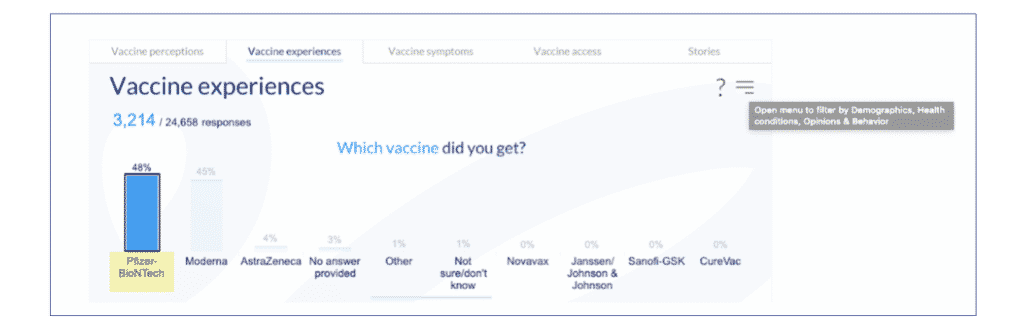
1. Go to the Vaccine experience tab, click on the “Pfizer/BioNTech” bar to see data only for those who got the vaccine from Pfizer/BioNTech.
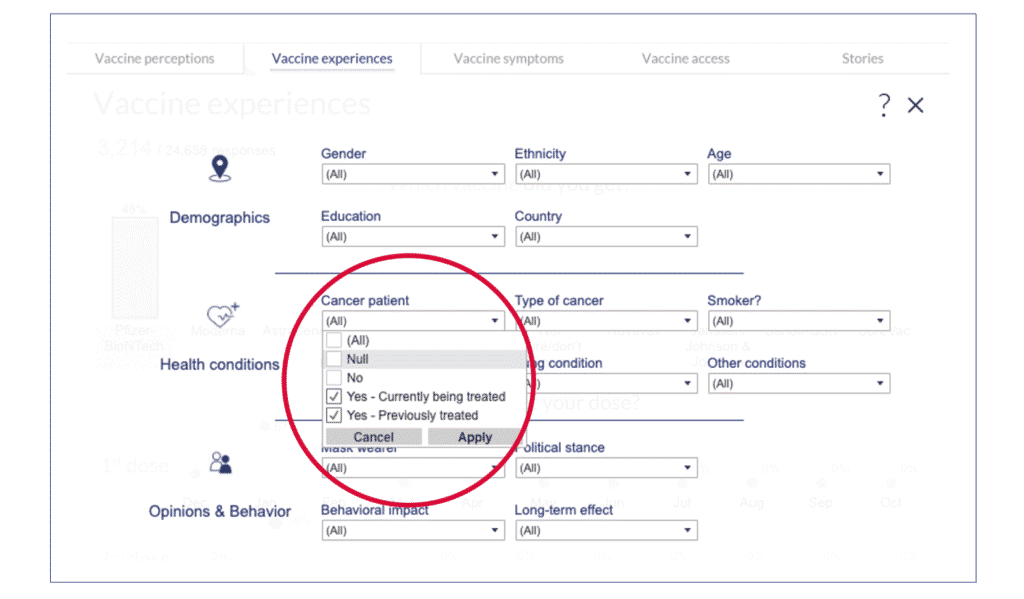
2. Open the global filter menu to access the filter menu.

3. Select just the cancer options and click “apply.” Click on “x” to exit the menu.
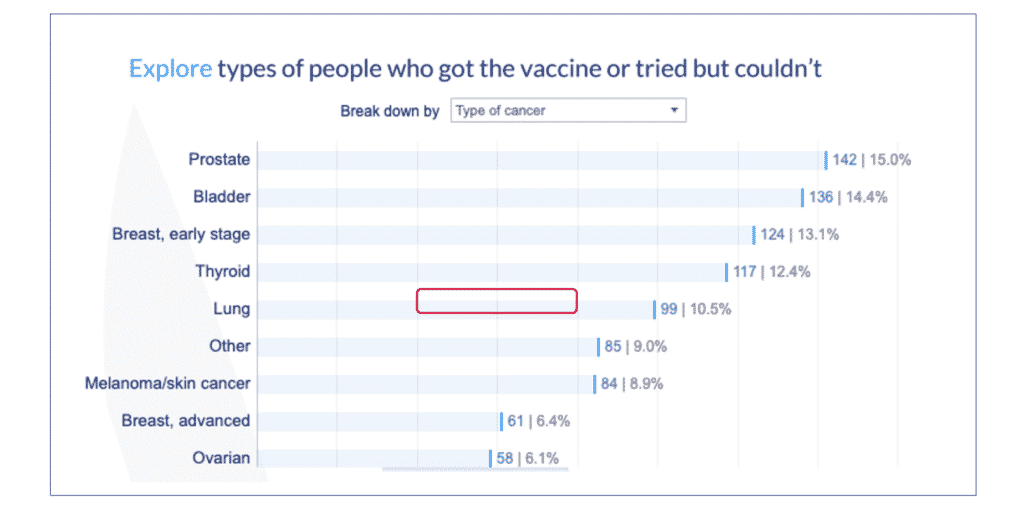
 4. To see the breakdown by cancer type go to the Vaccine experience tab again and click on “Breakdown by.” Choose “Type of cancer.”
4. To see the breakdown by cancer type go to the Vaccine experience tab again and click on “Breakdown by.” Choose “Type of cancer.”
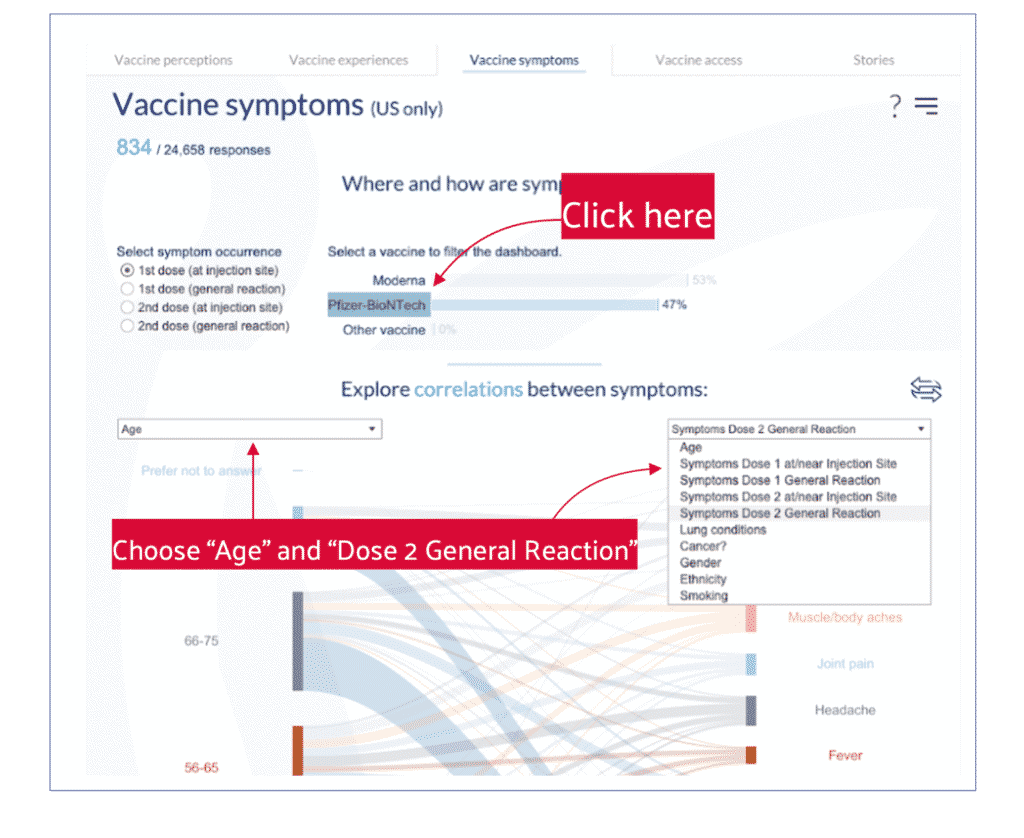
 5. Go to the Vaccine symptoms tab, click on “Pfizer/BioNTech,” and choose “Age and symptoms correlations.”
5. Go to the Vaccine symptoms tab, click on “Pfizer/BioNTech,” and choose “Age and symptoms correlations.”
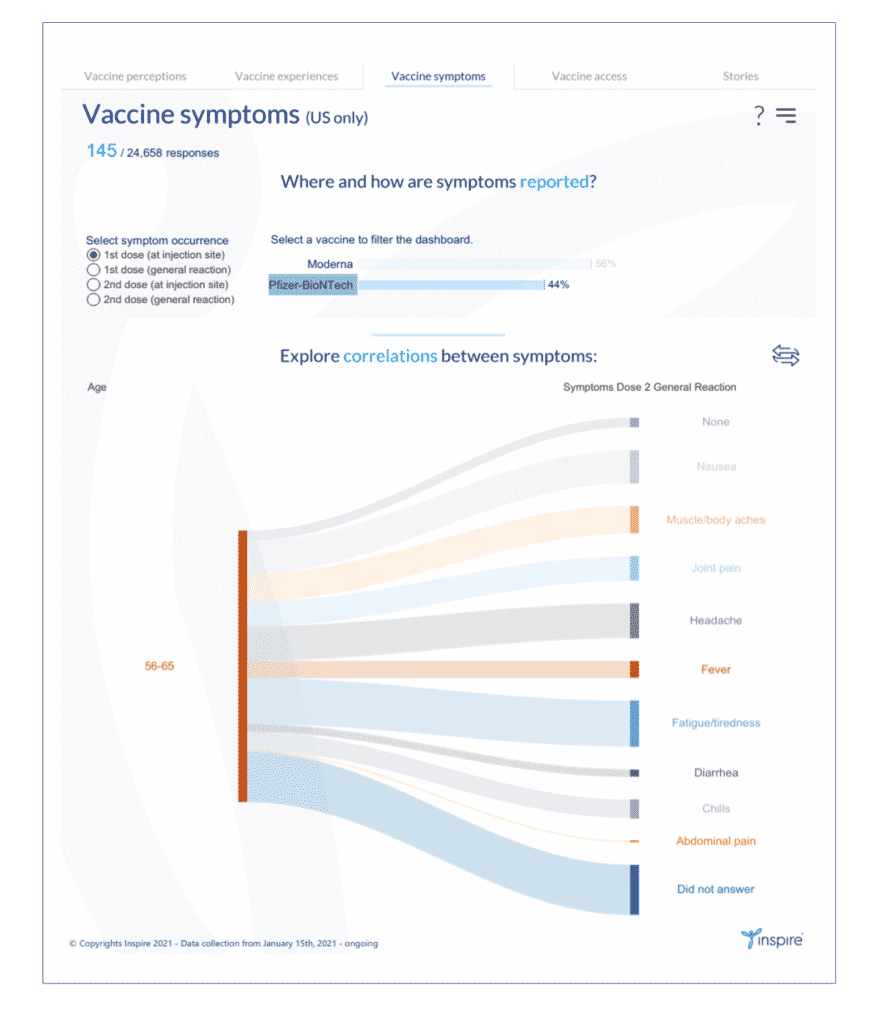
 6. Click on the global menu again on the upper right. Choose only “55-65” in the Age filter.
6. Click on the global menu again on the upper right. Choose only “55-65” in the Age filter.
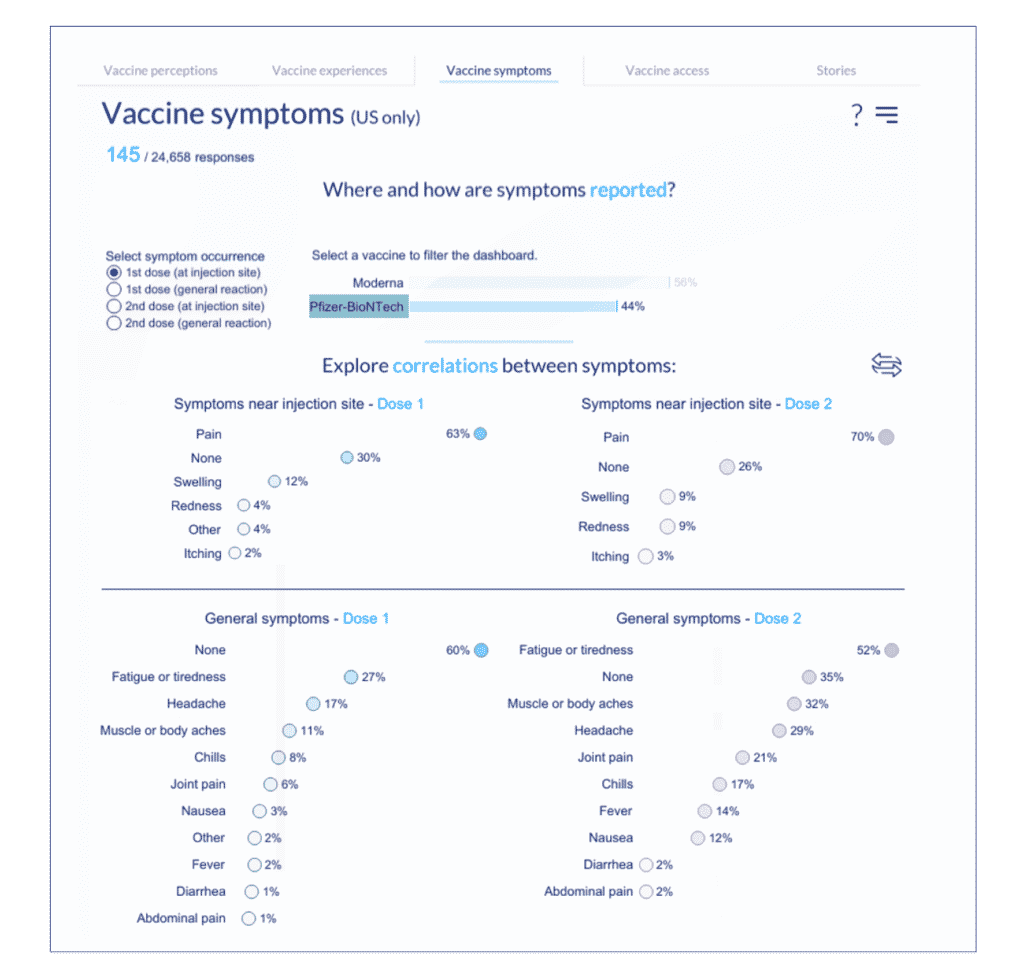
 7. Click on the chart visualization toggle (circular arrows) to change the visualization to show symptoms of all doses and type (both at injection site and general).
7. Click on the chart visualization toggle (circular arrows) to change the visualization to show symptoms of all doses and type (both at injection site and general).
 8. Click on the “Stories” tab to read stories that individuals provided to share publicly. Removing the age filter will leave just the stories written by people who have or have had cancer. Mouse over each dot around the world to quickly read what the person shared.
8. Click on the “Stories” tab to read stories that individuals provided to share publicly. Removing the age filter will leave just the stories written by people who have or have had cancer. Mouse over each dot around the world to quickly read what the person shared.
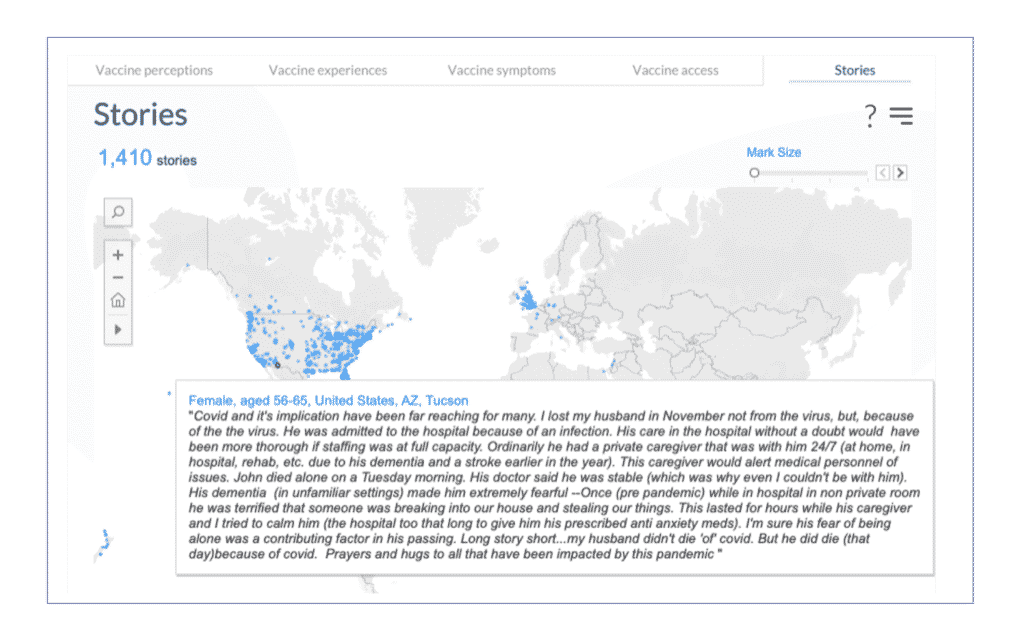 In summary, now you can see how people who have or have had cancer, between the age of 55-65, and received the Pfizer COVID-19 vaccine reported their symptoms.
In summary, now you can see how people who have or have had cancer, between the age of 55-65, and received the Pfizer COVID-19 vaccine reported their symptoms.
 As we mentioned in a Patient Pulse post, pharma’s key to success in 2021 depends on data integration that allows for holistic and longitudinal analysis of the patient experience. The COVID vaccine survey results reveal patient reported outcomes of treatment, unmet needs and treatment experiences. “Our goal is for both researchers and the public to have access to the same information in one place,” says Loew. “This kind of transparency is important so that everyone can make informed health decisions.”
As we mentioned in a Patient Pulse post, pharma’s key to success in 2021 depends on data integration that allows for holistic and longitudinal analysis of the patient experience. The COVID vaccine survey results reveal patient reported outcomes of treatment, unmet needs and treatment experiences. “Our goal is for both researchers and the public to have access to the same information in one place,” says Loew. “This kind of transparency is important so that everyone can make informed health decisions.”
Inspire researchers have always had nuanced access to the breadth of patient experiences through validated research tools such as this survey instrument, with over 2.2 million registered members generating tens of billions of data points across the words they have written, medical profiles, and other structured/unstructured data. Adding the COVID HealthJourney dashboard provides researchers access to a wide sample of people with varying health conditions including cancer, chronic lung diseases, autoimmune and rare diseases.
Over the next few months we will continue to track the safety and efficacy profiles of those who receive the vaccine and monitor changes in vaccine perceptions. Data is being collected on Post-Acute Sequelae of SARS-CoV-2 infection (PASC) or Long COVID and added to the dashboard. Follow-up pulse surveys are in the works.
Additionally, we are opening the survey to the general public in order to obtain further critical information on vaccine hesitancy, safety and Long COVID experiences.
We invite collaboration with academic and commercial research partners to further unlock discoveries. Please reach out to me directly at Richard@inspire.com if you are interested.
Inspire offers a trusted and vital community to patients and caregivers. Our goal with this blog, this website and our content is to provide the life science industry access to the true, authentic patient voice. In so doing, we support faithful operationalization of patient-centricity. Take a look at our case studies, eBooks and news outlet coverage.
References:






