Patient Engagement Leadership Series Part 2: Designing from the Patient Perspective

The patient perspective is based in the lived experience of illness. Entering into patient-centered or patient informed medication development means understanding that this worldview cannot be provided independently by researchers or clinicians.1
At Sanofi, patient perspectives have been integrated into clinical trials for over seven years and for more than five years in research, according to Victoria DiBiaso, Global Head Clinical Operations Strategy & Collaboration and her colleagues (Amy Ba, Patricia Roselle and Anthony Yanni). “It’s been a truly transformative approach and has helped us make smarter decisions.”
Benefits of Patient Informed Research & Development
There are multiple benefits to Sanofi’s approach. “Understanding medical value from the patient perspective in the pre-clinical space ensures we are investing into the science that will be most meaningful to patients,” Anthony Yanni, MD, Head, Medical Intelligence and Patient Perspectives, said. One advantage has been in the area of participant recruitment and retention.
Recruiting and Retention
Recruiting and retaining participants in clinical trials has been a persistent problem for the pharmaceutical industry. Consistently, studies reveal that barriers to patient involvement include fear of randomization 2 and of being experimented upon, a fear of losing privacy, a mistrust of researchers and worry over burdens like extra clinic visits, transportation costs, or costs not covered by insurance.3
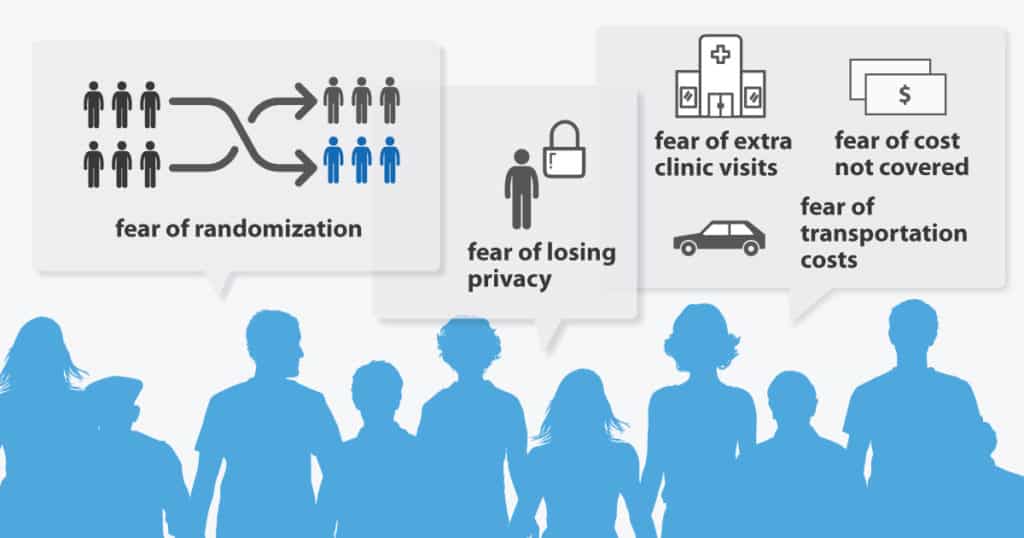
Yet other research on what supports recruitment include engaging prospective study participants in learning about their disease or illness.4 By involving patients in clinical trial development, Sanofi has avoided some of those pitfalls and increased awareness.
“When we design human clinical trials through the perspective of the patient community, we understand what elements in the study design will enable a patient to participate or deter them,” DiBiaso said.
Informed Consent
Poor understanding about what clinical trials are and what is necessary to participate is another barrier. Research participants often don’t understand basic concepts including the concept of randomization, placebo and risk.5,6,7
Additionally, the purpose of research may not be understood by clinical trial participants. Do patients realize that research is different from therapeutic clinical care? 7
Addressing these barriers is a challenge for the pharmaceutical industry, yet DiBiaso and her team see patient involvement as making a difference.
“We learn from patients what information is needed for them (and their families) to make a truly informed decision to participate and are able to close the educational and information gaps related to trial awareness and matching,” she said.
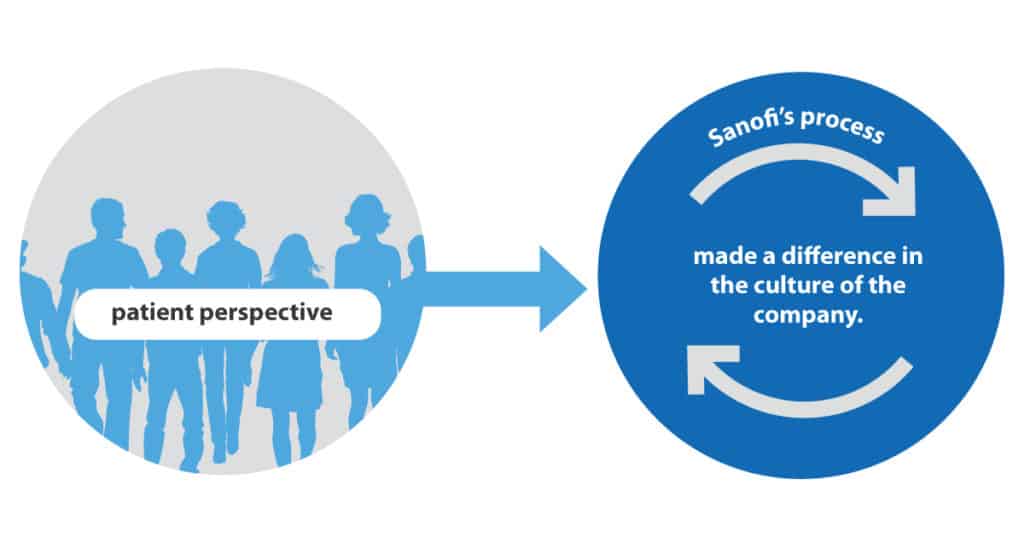
Changing Corporate Culture
Bringing the patient perspective into Sanofi’s process has made a difference in the culture of the company.
“When our study teams work with patient advisors, they are not only very grateful for the advice, but they also have a reinvigorated calling for why they go to work every day. Hoping that your program and/ or study could potentially impact the lives of thousands- or millions- of people is very motivating,” DiBiaso said.
Her team has also received positive feedback from patients who assist them.
“We hear routinely from patients that they want to be part of the drug development process and that when presented with opportunities to participate they enjoy helping to advance medical science,” she said.
The Bottom Line
Finally, Patient Informed Research and Development (R&D) at Sanofi positively affects the company’s bottom line.
“We are seeing our time to development reduce year over year; largely due to understanding the enablers of study conduct from a patient’s perspective,” DiBiaso said.
Patient Informed R&D “helps facilitate faster clinical trial recruitment which is the key driver of overall development timelines. The faster the studies recruit, the faster we will have the data to analyze and submit to regulators. Patient-informed clinical research is directly linked to improving overall R&D timelines.”
Inspire offers a trusted community to patients and caregivers. Our goal with this blog, this website and our content is to provide the life science industry access to the true, authentic patient voice. In so doing, we support faithful operationalization of patient-centricity. Take a look at our case studies, eBooks and news outlet coverage.
Reference:
1http://jamanetwork.com/journals/jama/fullarticle/1901303
2 https://ethndis.org/edonline/index.php/ethndis/article/view/510
3 https://trialsjournal.biomedcentral.com/articles/10.1186/1745-6215-15-399
4 http://journals.plos.org/plosmedicine/article?id=10.1371/journal.pmed.1000368
5https://www.ncbi.nlm.nih.gov/pmc/articles/PMC3539038
6 https://www.ncbi.nlm.nih.gov/pubmed/25130209
7 N Engl J Med 2015;372:855-62. DOI: 10.1056/NEJMra1411250 http://skateboardingalice.com/papers/2015_Grady.pdf