Patient Engagement Leadership Series
Part 1: Clinical Trial Participants Are Medical Heroes

“I think a hero is any person really intent on making this a better place for all people.” ~ Maya Angelou
In Embracing the Patient Centric Culture, I discussed the paradigm shift facing healthcare.
“Instead of doing things for or to the patient, now the goal must be empowering and working in partnership with the patient…to elevate the perspectives of patients and caregivers, incorporating their needs and priorities…. Building active listening to, or obtaining a continuous stream of insights from patients and caregivers into all aspects of product development must become standard for the pharmaceutical industry. Every person in the company needs to understand how their job impacts patients and needs to assimilate this information into their work ethic.”
How is this cultural change manifesting? As we look toward 2020 and beyond, I asked patient engagement leaders in the life science industry to share their experiences with patient partnership and their expectations for the future.
“From a clinical trial perspective, I’m hopeful that pharma will consistently begin to work in a model whereby R&D is conducted with patients more so than for patients,” said Victoria DiBiaso, Global Head Clinical Operations Strategy & Collaboration and her colleagues at Sanofi (Amy Ba, Patricia Roselle and Anthony Yanni). “Bringing clinical research directly to patient communities for collaborative input is essential if we’re going to reduce the time it takes to develop a new medicine.”
Addressing participation rates
In a recent evaluation of cancer clinical trial participation between 2000 and 2011, 18 percent of the trials closed after three years or more of recruitment due to low numbers of participants.1 DiBiaso and her team believe that, though historically patient participation rates are very low, the reasons behind this trend are reversible.
Collaborating with patient groups helped DiBiaso and the members of the Sanofi team design their research in ways that brought down barriers to increase participation.
“[Patients] have advised us on how to reduce the challenges [they] would face if entering a given clinical trial and provided logistical solutions to help facilitate patient participation,” she said.
Some of these solutions were reducing trial complexity and providing in-home options for trial participation. Additionally, trials can now leverage smartphone apps and wearables that patients might already own to collect trial data. By incorporating patient ideas, “We have witnessed a year-over-year reduction in the time [it takes] to conduct our studies,” DiBiaso said.

Sanofi’s patient network team also recommends working with patients to spread the word about clinical research opportunities.
“We hear from patients that they struggle to find studies that are recruiting in their community. Creating mechanisms for patients to easily know what options are available to them would be a significant achievement,” she said.
Motivating participation in clinical research
Better treatments and benefits to others are often what drive patients to participate in clinical trials.
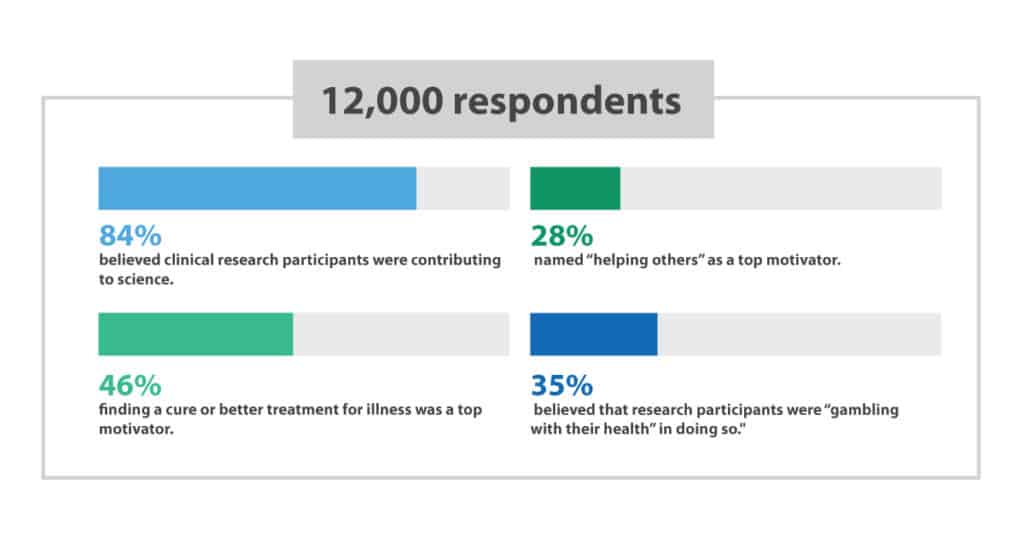
A global survey with over 12,000 respondents found that 84% believed clinical research participants were contributing to science. Forty six percent of respondents thought finding a cure or better treatment for illness was a top motivator while 28% named “helping others” as a top motivator. However, 35% of the surveyed group in the first trial believed that research participants were “gambling with their health” in doing so. 2
These results illustrate the work that needs to be done in explaining clinical research to the general public. DiBiaso and her team would “like to see the industry work together to truly make clinical research a positively understood household term. Educating the public and recognizing clinical trial participants as medical “heroes” should be a priority.”
Perhaps industry acknowledgement of patient participants would make a difference.
“People who contribute to clinical research through participation are the reason we are able to find new therapies and understand the causes of diseases; we need to do more to recognize their contributions to society,” DiBiaso said.
Patient Engagement Leadership Series
Our communication with patient engagement leaders has just begun. Look for future posts sharing insights from patient-centric industry leadership.
Inspire offers a trusted community to patients and caregivers. Our goal with this blog, this website and our content is to provide the life science industry access to the true, authentic patient voice. In so doing, we support faithful operationalization of patient-centricity. Take a look at our case studies, eBooks and news outlet coverage.