Updates from ASCO and ESMO 2021 Add to Successes in Hodgkin Lymphoma Research and Treatment
By Kathleen Hoffman, PhD, MSPH
In 1832 Sir Thomas Hodgkin described a morbid condition of the glands and spleen which in 1865 was named Hodgkin disease by Samuel Wilkes. It was a fatal disease. Yet it has become, as this Inspire patient describes, highly treatable.1
my first oncologist, trying to reassure me and make me feel better, told me that if someone said to him it was DEFINITE that he had to get cancer, but he could choose what kind, he would, without a doubt, pick Hodgkins Lymphoma …. for the very reason that it is so receptive to treatment and so highly curable.
Hodgkin lymphoma (HL) is a rare cancer in the US with around 9000 people expected to be diagnosed in 2021. A little over 1300 people die each year from this cancer but over 152,000 people are living with or in remission from HL. HL presents mostly during adolescence and early adulthood but it also occurs in adults over 50. 2
Typical symptoms, described by an Inspire member, include,
a low grade fever … usually around 100 degrees. I had chills and deep body aches …. I … felt like I was coming down with a bad flu… I had bad night sweats …. sometimes my pajamas and the sheets would be wet. I had a few places with weird red rashes that were very, very itchy …. on my calves, shins & feet, the backs of my upper arms and on my tailbone … Also, had a fair amount of weight loss …. wasn’t skeletal, but became thin enough for people … to start commenting on it. My appetite and interest in food decreased. I had a lot of fatigue, weakness and exhaustion at times and started sleeping WAY MORE than normal …. about 12 hours a day. Also, I had fairly large, swollen lymph nodes in my neck, armpits and along my collarbone.
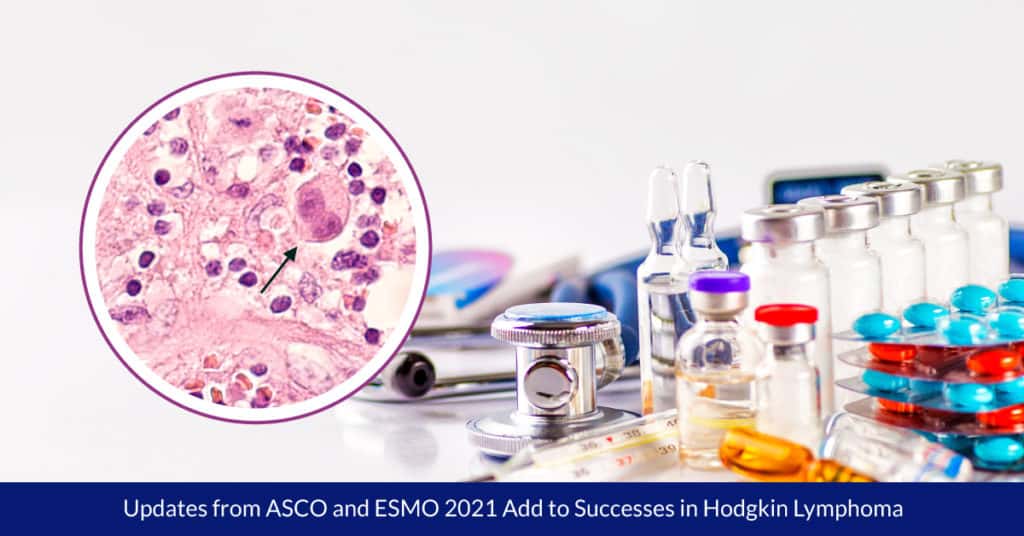
The cancer usually starts in the lymph nodes, often spreading to other nodes and to other organs. Definitive diagnosis includes a biopsy of lymph nodes and a hematopathology work up to identify the large B cells called Reed-Sternberg cells (named for the two scientists who discovered them in the 1900s[1]) that distinguish the cancer as HL. The two types of HL are classic (95 percent of HL diagnoses) and nodular lymphocyte-predominant HL (NLPHL), a slower growing form of HL.3
The transformation of HL from an always fatal cancer to one that can be routinely put into complete remission or even “cured” makes it one of the great success stories in cancer treatment. However, past treatments— high dose radiation and chemotherapies — have come at a high cost in the form of cardiovascular disease, infertility and sterility and subsequent secondary cancers.1 Discussions of long term toxic effects of early treatments can be found on Inspire. One family member shared her mother’s story:
I would like to share my Mom’s experiences with you: She was diagnosed with HL in the early 70’s, she was 16 at the time. It was considered 100% fatal at that time but cobalt radiation was cutting edge and an option to slow progression and give the patient some years to live. They told her she’d never be able to have children and she’d be LUCKY if she lived to see her 25th birthday. She passed just before her 59th birthday… More than twice the life expectancy she’d been promised. Over the years she had barely-controlled asthma, severe allergies, breast cancer twice, congestive heart failure (with multiple open heart surgeries to try to repair/ replace valves), and a blood clotting disorder. She also had 2 children, went back to college in her 40’s, and had a successful career as an RN (from which she retired after more than 30 years in the field). I tell you all of this because she had MANY great years, in spite of her residual medical issues after radiation. Before they discovered the cobalt worked, everyone died from HL. The treatments give you back your life.
To address these costs, research continues. Here are a few studies from American Society of Clinical Oncology (ASCO) 2021 and the European Society for Medical Oncology (ESMO) Congress 2021, addressing long term toxic effects and describing advances in new targeted therapies.
New research addresses long term side effects
Heart problems and breast cancer from radiation treatment are still two of the long term side effects that researchers are working to reduce. In a study presented at ASCO 2021, patients received two cycles of the chemotherapeutic regimen ABVD (doxorubicin, bleomycin, vinblastine and dacarbazine) then underwent a PET scan. If their disease showed less uptake of the radiotracer than their liver on their PET scan, they did not receive radiation therapy (78 percent of the patients) but did go through four more cycles of ABVD. The rest of the patients (22 percent) received escBEACOPP (bleomycin, etoposide, doxorubicin, cyclophosphamide, vincristine, procarbazine, prednisone) plus radiation. The study found the estimated progression-free survival for the no radiation group was 93.1 percent versus 89.7 percent for the radiation group and estimated 3-year survival was 98.6 percent for no radiation and 94.4 percent for the radiation group.4
Extending the use of novel treatments to younger populations
Today, treatment for HL, as with other cancers, has evolved from chemotherapy and radiotherapy to include immunotherapy. Stem cell transplantation, both autologous and allogeneic, is a further option available to patients.
Reed-Sternberg cancer cells make excellent targets for immunotherapies including monoclonal antibodies, antibody-drug conjugates, and checkpoint inhibitors because of the CD30 markers on the cells surface and the presence of PD-L1 and PD-L2 overexpressed on these cells due to chromosomal alterations.2
One treatment, Brentuximab vedotin, is an antibody-drug conjugate that has been approved for adults with HL. The monoclonal antibody part of the conjugate, brentuximab, targets the CD30 markers on the Reed-Sternberg cancer cells. Then the chemotherapeutic drug, vedotin, acting as a microtubule disruptor, prevents the cells from multiplying and leads to cell death.5 It is such an effective treatment for adults that a recent study was conducted on 77 pediatric patients who were stage IIB, IIIB and IV using brentuximab vedotin. Thirty-five patients obtained complete remission without the use of radiotherapy. The rest received radiotherapy. At the three year mark, event free survival was 97.4 percent, with overall survival at 98.7 percent.5 The study suggests that brentuximab can be used effectively not only for adults but also in pediatric patients.
Recent research for patients with refractory HL
As many as 40 percent of patients cannot undergo stem cell transplant or do not go into remission after autologous stem cell transplant. These patients with refractory HL need other options. Checkpoint inhibitors that target PD-L1 and PD-L2 are being investigated, especially focusing on relapsed or refractory HL. An interim report on the Keynote 204 phase III trial was recently published. This trial compares brentuximab vedotin with pembrolizumab for long term treatment, with “pembrolizumab showing a statistically significant and clinically meaningful improvement in progression-free survival compared with brentuximab vedotin, with a median of 13.2 months in the pembrolizumab arm (95% confidence interval [CI] = 10.9–19.4 months) compared with 8.3 months in the brentuximab vedotin arm (95% CI = 5.7–8.8 months; hazard ratio = 0.65, 95% CI = 0.48–0.88, P = .0027).” The study suggests that pembrolizumab can be an intermediate treatment helping patients to move forward to autologous or allogeneic stem cell transplant or even CAR T therapy.6
Finding new targeted treatments
Studies of two newer anti-PD-1 monoclonal antibodies have been presented as well. One study of penpulimab involved 94 patients with relapsed or refractory HL. Eighty-five patients had a response, with 40 patients obtaining a complete response after a median follow-up of 15.8 months.7 The other study used tislelizumab, another anti-PD-1 monoclonal antibody as a monotherapy in 70 patients who had not responded to autologous stem cell transplant or could not do stem cell transplant. Sixty-seven percent had a complete response at median follow-up of 33.8 months. Median progression free survival was 31.5 months.8 These two treatments could add to the arsenal of clinical targeted options that oncologists can offer patients with relapsed or refractory HL.
CAR-T cell therapy could offer even more options
A phase I/II clinical trial using Chimeric antigen receptor (CAR) T-cell therapy targeting CD30 on the Reed-Sternberg cells has been studied for patients with refractory or relapsed HL. Forty-one patients received the CAR T-cells. Seventy-two percent responded to the treatment with 19 patients (59 percent) obtaining complete remission. The safety profile for this phase I/II study was described as “excellent.” 9
Exploring tumor microenvironments prior to treatment?
Two other studies published in the last few months looked at the microenvironments of HL tumors to identify features that respond better to nivolumab. One study looking at 61 primary and 15 repeated tumor samples, found that biopsied tumors with lower levels of M2 macrophages had significantly greater likelihood of obtaining a complete response using nivolumab. Additionally, those samples with a low number of cancer cells that were CD163 positive had poorer results with nivolumab.10 Another study looked at interleukin-6 levels in the blood serum of HL patients and found that those with lower levels of interleukin-6 (lower than 2.2 pg/mL) had a better response to nivolumab, with higher numbers of patients having progression-free survival.11 These studies indicate the importance of taking the microenvironments of HL tumors into account when choosing treatments.
As Sandra Horning, in the 50 anniversary compilation of research for the American Society of Hematology, noted, the Hodgkin lymphoma “success story … created optimism for the treatment of cancer in general.”1 This recent research continues that optimism, highlighting the fact that research is continuing to push forward, addressing toxicity and providing more options for treatment. This means that the promise of a cure for HL, without long term repercussions from treatment, is ever closer for patients.
*******************************************************************************************************************************************************
Thursday, October 14 at 2pm ET Inspire presents, “Leveraging real world patient generated data to build clinical, commercial, and medical strategies.” If you can’t attend, register anyway for the recording. Hope to see you there!
Inspire offers a trusted community to patients and caregivers. Our goal with this blog, this website and our content is to provide the life science industry access to the true, authentic patient voice. In so doing, we support faithful operationalization of patient-centricity. Take a look at our case studies, eBooks and news outlet coverage.
References:
1Horning, MD,(2008) Milestones in Hodgkin Lymphoma, 50 Years in Hematology: Research That Revolutionized Patient Care. American Society of Hematology.Retrieved from https://www.hematology.org/about/history/50-years/milestones-hodgkin-lymphoma.
2https://lymphoma.org/wp-content/uploads/2018/03/HL-Booklet_2018.pdf
3https://www.cancer.org/cancer/hodgkin-lymphoma/about/what-is-hodgkin-disease.html
4https://ecancer.org/en/news/20331-asco-2021-pet-scans-following-initial-treatment
5Metzger ML, Link MP, Billett AL, et al. Excellent outcome for pediatric patients with high-risk Hodgkin lymphoma treated with brentuximab vedotin and risk-adapted residual node radiation. J Clin Oncol. Published online April 7, 2021. doi:10.1200/JCO.20.03286
6https://ascopost.com/issues/may-25-2021/pembrolizumab-as-long-term-treatment-option-in-relapsed-or-refractory-classic-hodgkin-lymphoma/
7Song Y, Zhou K, Jin C, et al. A phase II study of penpulimab, an anti-PD-1 antibody, in patients with relapsed or refractory classic Hodgkin lymphoma (cHL). J Clin Oncol. 2021;39:(suppl 15; abstr 7529). doi:10.1200/JCO.2021.39.15_suppl.7529
8Tislelizumab (BGB-A317) for relapsed/refractory classical Hodgkin lymphoma: long-term follow-up efficacy and safety results from a phase 2 study. Paper presented at: European Hematology Association 2021 Virtual Congress; June 2021; Abstract S207.
9https://ascopubs.org/doi/abs/10.1200/JCO.20.01342?journalCode=jco
10Fedorova L, Gusak A, Lepik K, et al. Immune microenvironment in classical Hodgkin lymphoma: composition and dynamics in patients with relapsed/ refractory disease. Presented at: European Society for Medical Oncology (ESMO) Congress 2021; September 16-21, 2021. Abstract 832MO
11Chekalov A, Shmidt D, Lepik K, et al. Role of assessment of IL-6, IL-15 and soluble PD-L1 levels as prognostic and predictive biomarkers in nivolumab-treated relapsed/refractory Hodgkin lymphoma. Presented at: European Society for Medical Oncology (ESMO) Congress 2021; September 16-21, 2021. Abstract 834P.






