The Patient Is Speaking: 5 Benefits of Patient Focused Drug Development Sponsors and CROs Need to Know

Since the 2012 FDASIA reauthorization of the Prescription Drug User Fee Act (PDUFA) and the 21st Century Cures Act ‐‐ Directing and Enabling Patient Focused Drug Development in 2016, integrating the patient perspective has been a priority. Our research team has conducted studies with patient and caregiver Inspire members for a variety of sponsors. I’d like to describe how these research projects produce important patient insights that inform and can improve clinical trials.
Patient focused drug development can
1) Help optimize clinical trial protocol and design
Patients can tell sponsors what will reduce interest in a clinical trial. In one study, Inspire’s research team conducted interviews and surveys for a client with potential clinical trial participants. As a part of the research, patients reviewed a protocol design. To be diagnosed with their condition, all of the patients had undergone a biopsy that confirmed their diagnosis. As they reviewed the clinical trial protocol, they found that the trial would require another biopsy. Patients indicated that a repeat biopsy would be a barrier to their participation in this trial. Their recommendation was acceptance of previous testing, like their diagnostic biopsy, instead of requiring the painful procedure again.
Patients did offer ways to make the possibility of having the biopsy more acceptable. They suggested that the clinician should provide explanations like understanding the results of the biopsy and knowing why biopsies are important would improve the experience. They also discussed better pain management, either being sedated or given something to help relax. The anxiety over the procedure increased the experience of pain, some thought. Others felt that having the procedure performed as an outpatient procedure did not allow enough time for recovery.
Additionally, patients asked that they be provided with two flyers, one that they can use to communicate information about the trial to their doctor and another version that they can give to their loved ones. Patients who had a trusted physician wanted that physician to be the study doctor or, if that was not possible, have the study doctor coordinate with their personal physician.
2) Optimize trial planning and support feasibility
Increasing the feasibility of a trial can be as simple as finding out the barriers to enrollment. One barrier for minorities to participate in newer immunotherapies requires patients to have Next Generation Sequencing of their tumors. When this barrier is removed, as described in a study published in 2018 in the Journal of Clinical Oncology, a greater number of minority participants can engage in this type of clinical research. 1
Patients experiencing different conditions have different needs. For example, in research our team conducted with patients with an advanced cancer, the length of the trial was important. For another condition, patients did not want to travel, indicating that they would be willing to travel only 25 miles or less.
In November 2018, Inspire sent a clinical trial perceptions research survey to caregiver and patient members with the following conditions: ovarian, colorectal and prostate cancer; scleroderma and sarcoidosis; and psoriasis and arthritis. The research – discussed in a webinar last week – had over 1600 respondents. One set of Likert scale questions asked, “How would the following factors influence your likelihood to participate in a clinical trial?
Likert scale answers:
Much more likely to participate (4)
More likely to participate (3)
No influence (2)
I would be less likely to participate (1)
In the table below, it is apparent that consulting with an expert is important to all the conditions but especially to those with rare conditions or ovarian cancer. Additionally, being able to stay on the medication in the trial for as long as it works for you as well as having a current doctor do most of the tests and clinic visits is more important for women diagnosed with ovarian cancer. Having transportation available is more important for people living with scleroderma.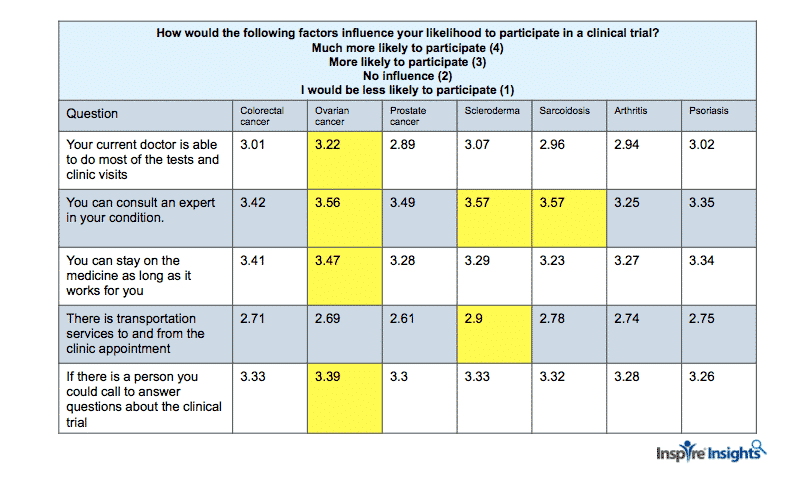
3) Accelerate enrollment
Recent research at Inspire informed a client of the influence patient experiences can have on enrollment into clinical trials. Inspire compared two groups of patients with two different types of advanced cancer. Group one obtained their diagnosis with relative ease. For Group two, patients reported multiple misdiagnoses and referrals as well as a circuitous path to diagnosis.
That difference in diagnoses experience influenced the trajectory of these two groups treatment experiences, support and decision-making. Group one knew others with their cancer, and knew that their diagnosis was very serious and life threatening. In contrast, Group two had limited information about their cancer, knew few people with the cancer and dealt with stigma around their cancer.
Group one’s knowledge level made them eager to uncover all their treatment options including researching clinical trials. Group two followed their physician’s advice but were told they had limited options, most had not been offered a clinical trial and did not know how to search for a clinical trial.
Having these details about Group two, provided sponsors with information they did not have and allowed them to develop strategies to accelerate enrollment with these patients.
4) Increase retention
In a study conducted by Inspire, patients with a serious chronic progressive condition reported that receiving information on their test results, receiving information about their condition, receiving a newsletter on the progress of the clinical trial and receiving appointment reminders would be influential in their interest in staying on a trial.
In this same study, patients wanted to be kept informed and they also wanted the researchers who run the study to realize that they are trying to continue their jobs and lives while on a clinical trial. Often the trial is given more importance than the people who participate in it, they felt. Those running the trial do not express empathy or seem to understand the costs to patients of mistakes, delays or miscommunications which may require the patient to take time from work or other parts of their lives. Patients ask to please keep them informed so that they don’t feel like lab rats.
For patients with serious, often fatal, conditions, issues around quality of life in a clinical trial influence their interest in staying with a clinical trial. In a study conducted for a client, patients and caregivers wanted to know risks, if the trial had a placebo and the potential side effects as well as invasive testing like biopsies. Retention in a trial of this nature might depend on researchers coming to the patient or using telemedicine or other technology to monitor patients who are fragile.
5) Increase understanding of a condition
The FDA defines Patient Focused Drug Development as “a systematic approach to help ensure that patients’ experiences, perspectives, needs and priorities are captured and meaningfully incorporated into drug development and evaluation.” Over the course of three years, FDA held and led 24 Patient Focused Drug Development Disease Area Meetings called “Voice of the Patient” (VOP). At these meetings patient advocates came to the FDA and answered questions, shared insights and experiences. FDA transcribed these meetings. In 2018, Inspire worked with the FDA to compare the transcripts of one of the VOP meetings on a debilitating and fatal rare condition with unstructured user-generated data by Inspire members with the same condition. The FDA had three fold interest 1) structure the two data sets, 2) identify similarities and differences in the data sets and see if, 3) the combination of the two datasets would provide a more comprehensive description of the patient experience.
Inspire’s research team used Natural Language Processing and iterative hands-on manual linguistic curation to identify key themes and learnings from both data sets. Patients on Inspire were at an earlier stage in the patient journey than advocates addressing the FDA. At this early stage, patients did not realize the seriousness of the disorder and focused on other comorbidities, even asking others if they really needed to take the medication prescribed for the rare condition. Patients discussed quality of life on Inspire but they were also trying to gain an understanding of this new diagnosis. Patients at the VOP meeting were at a later stage of their journey. They had adjusted to their diagnosis in the sense of realizing what limitations the disease placed on them. Their discussion focused on their quality of life and how the condition impaired normal everyday functioning, impacting normal life in fundamental ways.
Together, analysis of two unstructured data sets, provided a rich and comprehensive description of the patients’ journey with this rare fatal condition emerged. 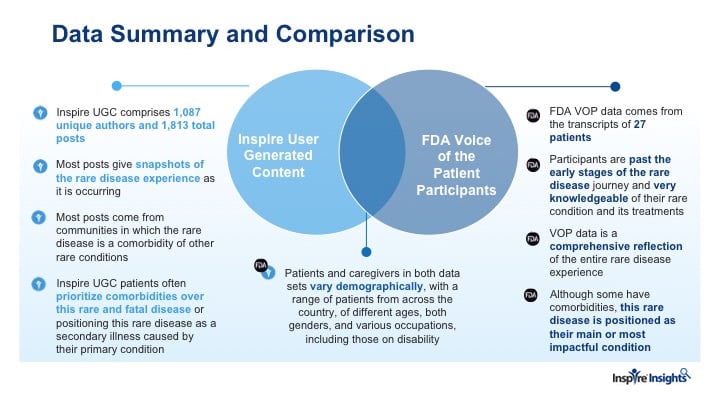
Incorporating insights from the people who are volunteering and are most impacted by a clinical trial essentially adds important missing data points to design and development of clinical trial protocols. FDA continues its efforts -through developing guidance documents- to increase the comprehensive understanding of the patient voice as well as include patient perspectives in drug development.
As FDA fulfills its regulatory mission, sponsors can rely on Inspire to provide needed research to improve their clinical trials.
Inspire offers a trusted community to patients and caregivers. Our goal with this blog, this website and our content is to provide the life science industry access to the true, authentic patient voice. In so doing, we support faithful operationalization of patient-centricity. Take a look at our case studies, eBooks and news outlet coverage.
References:
1 Cotta, J. Gordon, B, Trent J. (2018). Increasing access to NGS tests for women and racial minorities to lift barriers to molecular driven clinical trial enrollment. Journal of Clinical Oncology. https://ascopubs.org/doi/abs/10.1200/JCO.2018.36.15_suppl.6561